Silver Doping Mechanism in Bioceramics—From Ag+:Doped HAp to Ag°/BCP Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sol-Gel Elaboration of Silver Containing BCP Samples
2.2. Powder X-Ray Diffraction (PXRD) and Rietveld Analyses
2.3. Scanning Electron Microscopy (SEM)
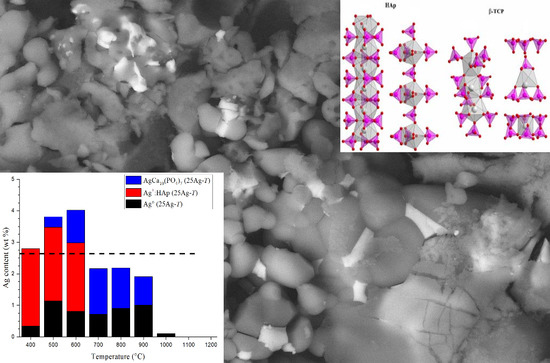
3. Results: Materials Characterization
3.1. Quantitative Phase Analysis
3.2. Thermal Variation in the HAp Structural Parameters
3.3. Thermal Variation in the β-TCP Structural Parameters
3.4. Ag Location Studied by SEM Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dorozhkin, S.V. Biocomposites and hybrid biomaterials based on calcium orthophosphates. Biomaterials 2011, 1, 3–56. [Google Scholar] [Green Version]
- Dahl, S.G.; Allain, P.; Marie, P.J.; Mauras, Y.; Boivin, G.; Ammann, P.; Tsouderos, Y.; Delmas, P.D.; Christiansen, C. Incorporation and distribution of strontium in bone. Bone 2001, 8, 446–453. [Google Scholar] [CrossRef]
- Lagier, R.; Baud, C.A. Magnesium whitlockite, a calcium phosphate crystal of special interest in pathology. Pathol. Res. Pract. 2003, 199, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.S.; Kayser, M.V.; Ali, S.Y. Calcium phosphate microcrystal deposition in the human intervertebral disc. J. Anat. 2006, 208, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Combes, C.; Drouet, C.; Glimcher, M.J. Bone mineral: Update on chemical composition and structure. Osteoporos. Int. 2009, 20, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Cazalbou, S.; Combes, C.; Eichert, D.; Rey, C. Adaptative physico-chemistry of bio-related calcium phosphates. J. Mater. Chem. 2004, 14, 2148–2153. [Google Scholar] [CrossRef]
- Elliot, J.C. Structure and Chemistry of the Apatite and Other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Turkoz, M.; Atilla, A.O.; Evis, Z. Silver and fluoride doped hydroxyapatite: Investigation by microstructure, mechanical and antibacterial properties. Ceram. Int. 2013, 39, 8925–8931. [Google Scholar] [CrossRef]
- Diaz, M.; Zia, R.; Sameemi, F.; Ikram, H.; Bashir, F. In vitro antimicrobial activity of ZnO based glass-ceramics against pathogenic bacteria. J. Mater. Sci. Mater. Med. 2015, 26, 268. [Google Scholar]
- Khan, M.S.; ur Rehman, S.; Ali, M.A.; Sultan, B.; Sultan, S. Infection in orthopedic implant surgery, its risk factors and outcome. J. Ayub Med. Coll. Abbottabad 2008, 20, 23–25. [Google Scholar]
- Cremet, L.; Corvec, S.; Bemer, P.; Bret, L.; Lebrun, C.; Lesimple, B.; Miegeville, A.F.; Reynaud, A.; Lepelletier, D.; Caroff, N. Orthopaedic-implant infection by Escherichia coli: Molecular and phenotypic analysis of the causative strains. J. Infect. 2012, 64, 169–175. [Google Scholar] [CrossRef]
- Salwiczek, M.; Qu, Y.; Gardiner, J.; Strugnell, R.A.; Lithgow, T.; McLean, K.M.; Thissen, H. Emerging rules for effective antimicrobial coatings. Trends Biotechnol. 2014, 32, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Nasser, S. Prevention and treatment of sepsis in total hip replacement surgery. Orthop. Clin. N. Am. 1992, 23, 265–277. [Google Scholar]
- Li, P.L.; Zamora, J.; Bentley, G. The results at ten years of the Insall-Burstein II total knee replacement: Clinical, radiological and survivorship studies. J. Bone Joint Surg. Br. 1999, 81, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, J.G.E.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Background of antibiotic-loaded bone cement and prosthesis-related infection. Biomaterials 2004, 25, 545–556. [Google Scholar] [CrossRef]
- Fan, J.C.H.; Hung, H.H.; Fung, K.Y. Infection in primary total knee replacement. Hong Kong Med. J. 2008, 14, 40–45. [Google Scholar] [PubMed]
- Sygnatowicz, M.; Keyshar, K.; Tiwari, A. Antimicrobial properties of silver-doped hydroxyapatite nano-powders and thin films. Biol. Biomed. Mater. 2010, 62, 65–70. [Google Scholar] [CrossRef]
- Rauschmann, M.A.; Wichelhaus, T.A.; Stirnal, V.; Dingeldein, E.; Zichner, L.; Schnettler, R.; Alt, V. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotic in bone infections. Biomaterials 2005, 26, 2677–2684. [Google Scholar] [CrossRef]
- Baradari, H.; Damia, C.; Dutreih-Colas, M.; Laborde, E.; Pecout, N.; Champion, E.; Chulia, D.; Viana, M. Calcium phosphate porous pellets as drug delivery systems: Effect of drug carrier composition on drug loading and in vitro release. J. Eur. Ceram. Soc. 2012, 32, 2679–2690. [Google Scholar] [CrossRef]
- Clement, J.L.; Jarrett, P.S. Antibacterial silver. Metal. Based Drugs 1994, 1, 467–482. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nano Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Iqbal, N.; Kadir, A.R.M.; Malek, N.N.A.N.; Mahmood, H.N.; Murali, R.M.; Kamarul, T. Rapid microwave assisted synthesis and characterization of nanosized silver-doped hydroxyapatite with antibacterial properties. Mater. Lett. 2012, 89, 118–122. [Google Scholar] [CrossRef]
- Ning, C.; Wang, X.; Li, L.; Zhu, Y.; Li, M.; Yu, P.; Zhou, L.; Zhou, Z.; Chen, J.; Tan, G.; et al. Concentration ranges of antibacterial cations for showing the highest antibacterial efficacy but the least cytotoxicity against mammalian cells: Implications for a new antibacterial mechanism. Chem. Res. Toxicol. 2015, 28, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Feng, Q.L.; Kim, J.O.; Wu, J.; Wang, H.; Chen, G.C.; Cui, F.Z. Antimicrobial effects of metal ions (Ag+, Cu2+, Zn2+) in hydroxyapatite. J. Mater. Sci. Mater. Med. 1998, 8, 129–134. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Rameshbabu, N.; Sampath Kumar, T.S.; Prabhakar, T.G.; Sastry, V.S.; Murty, K.V.G.K.; Prasad Rao, K. Antibacterial nanosized silver substituted hydroxyapatite: Synthesis and characterization. J. Biomed. Mater. Res. A 2007, 80, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Klasen, H.J. Historical review of the use of silver in the treatment of burns: I. Early uses. Burns 2000, 26, 117–130. [Google Scholar] [CrossRef]
- Gosheger, G.; Hardes, J.; Ahrens, H.; Streitburger, A.; Buerger, H.; Erren, M.; Gunsel, A.; Kemper, F.H.; Winkelmann, W.; von Eiff, C. Silver-coated megaendoprostheses in a rabbit model—An analysis of the infection rate and toxicological side effects. Biomaterials 2004, 25, 5547–5556. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.S.; Iconaru, S.L.; Pasuk, I.; Vasile, B.S.; Lupu, A.R.; Hermenean, A.; Dinischiotu, A.; Predoi, D. Structural properties of silver doped hydroxyapatite and their biocompatibility. Mater. Sci. Eng. C 2013, 33, 1395–1402. [Google Scholar] [CrossRef]
- Jadalannagari, S.; Deshmukh, K.; Ramanan, S.R.; Kowshik, M. Antimicrobial activity of hemocompatible silver doped hydroxyapatite nanoparticles synthesized by modified sol-gel technique. Appl. Nanosci. 2014, 4, 133–141. [Google Scholar] [CrossRef]
- Fu, C.; Zhang, X.; Savino, K.; Gabrys, P.; Gao, Y.; Chaimayo, W.; Miller, B.L.; Yates, M.Z. Antimicrobial silver-hydroxyapatite composite coating through two-stage electrochemical synthesis. Surf. Coat. Technol. 2016, 301, 13–19. [Google Scholar] [CrossRef]
- Geng, Z.; Cui, Z.; Li, Z.; Zhu, S.; Liang, Y.; Liu, Y.; He, X.; Yu, X.; Wang, R.; Yang, W. Strontium incorporation to optimize the antibacterial and biological characteristics of silver-substituted hydroxyapatite coating. Mater. Sci. Eng. C 2016, 58, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Gokcekaya, O.; Webster, T.J.; Ueda, K.; Narushima, T.; Ergun, C. In vitro performance of Ag-incorporated hydroxyapatite and its adhesive porous coating deposited by electrostatic spaying. Mater. Sci. Eng. C 2017, 77, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, X.; Hai, J.; Li, T. Synthesis of silver-hydroxyapatite composite with improved antibacterial properties. Vacuum 2018, 152, 132–137. [Google Scholar] [CrossRef]
- Riaz, M.; Zia, R.; Ijaz, A.; Hussain, T.; Mohsin, M.; Malik, A. Synthesis of monophasic Ag doped hydroxyapatite and evaluation of antibacterial activity. Mater. Sci. Eng. C 2018, 90, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Dubnika, A.; Loca, D.; Rudovica, V.; Parekh, M.B.; Berzina-Cimdina, L. Functionalized silver-doped hydroxyapatite scaffolds for controlled simultaneous silver ion and drug delivery. Ceram. Int. 2017, 43, 3698–3705. [Google Scholar] [CrossRef]
- Zhang, X.; Chaimayo, W.; Yang, C.; Yao, J.; Miller, B.L.; Yates, M.Z. Silver-hydroxyapatite composite coatings with enhances antimicrobial activities through heat treatment. Surf. Coat. Technol. 2017, 325, 39–45. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ueda, K.; Narushima, T.; Ergun, C. Synthesis and characterization of Ag-containing calcium phosphates with various Ca/P ratios. Mater. Sci. Eng. C 2015, 53, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Kadir, M.R.A.; Mahmood, N.H.; Salim, N.; Froemming, G.R.A.; Balaji, H.R.; Kamarul, T. Characterization, antibacterial and in-vitro compatibility of zinc-silver doped hydroxyapatite nanoparticles prepared through microwave synthesis. Ceram. Int. 2014, 40, 4507–4513. [Google Scholar] [CrossRef]
- Kaygili, O.; Keser, S.; Dorozhkin, S.V.; Yakuphanoglu, F.; al-Ghamdi, A.A.; Kirbag, S.; Sertkaya, D.; Ates, T.; Gursoy, N.C. Structural and dielectrical properties of Ag- and Ba-substituted hydroxyapatites. J. Inorg. Organomet. Polym. 2014, 24, 1001–1008. [Google Scholar] [CrossRef]
- Liu, X.; Mou, Y.; Wu, S.; Man, H.C. Synthesis of silver-incorporated hydroxyapatite nanocomposites for antimicrobial implant coatings. Appl. Surf. Sci. 2013, 273, 748–757. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.; Xie, Y.; Ji, H.; Ding, C.; Li, H.; Dai, K. Silver release from silver-containing hydroxyapatite coatings. Surf. Coat. Technol. 2010, 205, 1892–1896. [Google Scholar] [CrossRef]
- Chen, W.; Oh, S.; Ong, A.P.; Oh, N.; Liu, Y.; Courtney, H.S.; Appleford, M.; Ong, J.L. Antibacterial and osteogenic properties of silver-containing hydroxyapatite coatings produced using sol-gel process. J. Biomed. Mater. Res. A 2007, 82, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Vukomanovic, M.; Bracko, I.; Poljansek, I.; Uskokovic, D.; Skapin, S.D.; Suvorov, D. The growth of silver nanoparticles and their combination with hydroxyapatite to form composites via a sonochemical approach. Cryst. Growth Des. 2011, 11, 3802–3812. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Courtney, H.S.; Bettenga, M.; Agrawal, C.M.; Bumgardner, J.D.; Ong, J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Kim, T.N.; Wu, J.; Park, E.S.; Kim, J.O.; Lim, D.Y.; Cui, F.Z. Antibacterial effects of Ag-HAp thin films on alumina substrates. Thin Solid Films 1998, 335, 214–219. [Google Scholar] [CrossRef]
- Shirkhanzadeh, M.; Azadegan, M.; Liu, G.Q. Bioactive delivery systems for the slow release of antibiotics: Incorporation of Ag+ ions into micro-porous hydroxyapatite coatings. Mater. Lett. 1995, 24, 7–12. [Google Scholar] [CrossRef]
- Renaudin, G.; Gomes, S.; Nedelec, J.-M. First-row transition metal doping in calcium phosphate bioceramics: A detailed crystallographic study. Materials 2017, 10, 92. [Google Scholar] [CrossRef]
- Gomes, S.; Nedelec, J.-M.; Renaudin, G. On the effect of temperature on the insertion of zinc into hydroxyapatite. Acta Biomater. 2012, 8, 1180–1189. [Google Scholar] [CrossRef] [Green Version]
- Gomes, S.; Kaur, A.; Nedelec, J.-M.; Renaudin, G. X-ray Absorption Spectroscopy shining (synchrotron) light onto the insertion of Zn2+ in calcium phosphate ceramics and its influence on their behaviour in biological conditions. J. Mater. Chem. B 2014, 2, 536–545. [Google Scholar] [CrossRef]
- Gomes, S.; Nedelec, J.-M.; Jallot, E.; Sheptyakov, D.; Renaudin, G. Unexpected mechanism of Zn2+ insertion in calcium phosphate bioceramics. Chem. Mat. 2011, 23, 3072–3085. [Google Scholar] [CrossRef]
- Gomes, S.; Kaur, A.; Grenèche, J.-M.; Nedelec, J.-M.; Renaudin, G. Atomic scale modeling of iron-doped biphasic calcium phosphate bioceramics. Acta Biomater. 2017, 50, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Vichery, C.; Descamps, S.; Martinez, H.; Kaur, A.; Jacobs, A.; Nedelec, J.-M.; Renaudin, G. Cu-doping of calcium phosphate bioceramics: From mechanism to the control of cytotoxicity. Acta Biomater. 2018, 65, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carvajal, J. PROGRAM FullProf.2k—Version 3.20; Laboratoire Léon Brillouin (CEA-CNRS): Saclay, France, 2005; FullProf.2k Manual; Available online: http://www-llb.cea.fr/fullweb/fp2k/fp2k_divers.htm (accessed on 24 June 2019).
- Strutynska, N.Y.; Zatovsky, I.V.; Ogorodnyk, I.V.; Slobodyanik, N.S. Rietveld refinement of AgCa10(PO4)7 from X-ray powder data. Acta Cryst. E 2013, 69, i23. [Google Scholar] [CrossRef] [PubMed]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of beta-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Sol. State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Owen, E.A.; Yates, E.L. Precision measurement of crystal parameters. Philos. Mag. 1933, 15, 472–488. [Google Scholar]
- Mocanu, A.; Furtos, G.; Rapuntean, S.; Horovitz, O.; Flore, C.; Garbo, C.; Danisteanu, A.; Rapuntean, G.; Prejmerean, C.; Tomoaia-Cotisel, M. Synthesis; characterization and antimicrobial effects of composites based on multi-substituted hydroxyapatite and silver nanoparticles. Appl. Surf. Sci. 2014, 298, 225–235. [Google Scholar] [CrossRef]
- Singh, B.; Dubey, A.K.; Kumar, S.; Saha, N.; Basu, B.; Gupta, R. In vitro biocompatibility and antimicrobial activity of wet chemically prepared Ca10−xAgx(PO4)6(OH)2 (0.0 ≤ x ≤ 0.5) hydroxyapatites. Mater. Sci. Eng. C 2011, 31, 1320–1329. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Badrour, L.; Sadel, A.; Zahir, M.; Kimakh, L.; el Hajbi, A. Synthesis and physical and chemical characterization of Ca10−xAgx(PO4)6(OH)2−xx apatites. Ann. Chim. Sci. Mat. 1998, 23, 61–64. [Google Scholar] [CrossRef]








| Sample | Phase Composition (wt %) | ||||
|---|---|---|---|---|---|
| HAp | TCP | CDP | Eq. CaO | Ag° | |
| 0Ag-400 | 97.1 (2.0) | 2.9 (1.0) | / | / | / |
| 0Ag-500 | 94.8 (2.0) | 1.5 (0.5) | 2.1 (0.5) | 1.6 (0.5) | / |
| 0Ag-600 | 91.8 (2.0) | 3.3 (1.0) | 3.8 (1.0) | 1.2 (0.5) | / |
| 0Ag-700 | 85.8 (2.0) | 9.3 (1.0) | 3.1 (1.0) | 1.9 (0.5) | / |
| 0Ag-800 | 81.9 (2.0) | 16.0 (1.0) | / | 2.1 (0.5) | / |
| 0Ag-900 | 81.4 (2.0) | 16.7 (1.0) | / | 1.9 (0.5) | / |
| 0Ag-1000 | 85.8 (2.0) | 13.6 (1.0) | / | 0.6 (0.5) | / |
| 0Ag-1100 | 92.5 (2.0) | 7.1 (1.0) | / | 0.4 (0.2) | / |
| 0Ag-1200 | 96.0 (2.0) | 3.8 (1.0) | / | 0.2 (0.2) | / |
| 25Ag-400 | 92.7 (2.0) | 2.5 (0.5) | 3.1 (1.0) | 1.2 (0.5) | 0.3 (0.2) |
| 25Ag-500 | 88.5 (2.0) | 3.6 (1.0) | 4.1 (1.0) | 2.6 (0.5) | 1.1 (0.5) |
| 25Ag-600 | 81.9 (2.0) | 11.3 (1.0) | 3.5 (1.0) | 2.4 (0.5) | 0.8 (0.5) |
| 25Ag-700 | 80.4 (2.0) | 15.8 (1.0) | 1.1 (0.5) | 2.2 (0.5) | 0.7 (0.5) |
| 25Ag-800 | 82.9 (2.0) | 14.0 (1.0) | / | 2.2 (0.5) | 0.9 (0.5) |
| 25Ag-900 | 87.1 (2.0) | 19.9 (1.0) | / | 2.0 (0.5) | 1.0 (0.5) |
| 25Ag-1000 | 93.1 (2.0) | 5.3 (1.0) | / | 1.5 (0.5) | 0.1 (0.2) |
| 25Ag-1100 | 96.8 (2.0) | 2.0 (0.5) | / | 1.2 (0.5) | / |
| 25Ag-1200 | 99.0 (2.0) | 0.5 (0.2) | / | 0.5 (0.2) | / |
| 100Ag-400 | 92.3 (2.0) | / | / | 2.4 (0.5) | 5.3 (1.0) |
| 100Ag-500 | 81.4 (2.0) | 7.4 (1.0) | 2.3 (0.5) | 3.4 (1.0) | 5.5 (1.0) |
| 100Ag-600 | 76.8 (2.0) | 12.5 (1.0) | 1.8 (0.5) | 3.0 (1.0) | 5.9 (1.0) |
| 100Ag-700 | 73.8 (2.0) | 16.1 (1.0) | 1.0 (0.5) | 3.2 (1.0) | 6.0 (1.0) |
| 100Ag-800 | 77.3 (2.0) | 13.9 (1.0) | / | 2.9 (0.5) | 5.9 (1.0) |
| 100Ag-900 | 79.4 (2.0) | 13.5 (1.0) | / | 1.4 (0.5) | 5.6 (1.0) |
| 100Ag-1000 | 85.5 (2.0) | 8.7 (1.0) | / | 1.1 (0.5) | 4.8 (1.0) |
| 100Ag-1100 | 92.2 (2.0) | 4.7 (1.0) | / | 0.4 (0.2) | 2.8 (0.5) |
| 100Ag-1200 | 96.8 (2.0) | 3.0 (1.0) | / | 0.1 (0.2) | / |
| Sample | Analysis | Atomic Composition (%) | Calculated Ratio | Expected Ratio | ||||
|---|---|---|---|---|---|---|---|---|
| Ca | Ag | P | Ag/Ca | Ca/P | Ag/Ca | Ca/P | ||
| 0Ag-400 | global | 65.63 | 0.00 | 34.37 | 0.00 | 1.91 | 0.00 | 1.67 |
| 0Ag-700 | global | 59.80 | 0.00 | 40.20 | 0.00 | 1.49 | ||
| 0Ag-1200 | global | 68.02 | 0.00 | 31.98 | 0.00 | 2.13 | ||
| 25Ag-400 | global | 66.47 | 2.17 | 31.37 | 0.03 | 2.12 | 0.03 | 1.62 |
| 25Ag-700 | global | 60.14 | 1.43 | 38.43 | 0.02 | 1.56 | ||
| 25Ag-1200 | global | 65.48 | 0.00 | 34.52 | 0.00 | 1.90 | ||
| 100Ag-400 | global | 62.39 | 3.94 | 33.68 | 0.06 | 1.85 | 0.11 | 1.50 |
| 100Ag-700 | global | 62.35 | 3.03 | 34.61 | 0.05 | 1.80 | ||
| 100Ag-1200 | global | 60.59 | 0.00 | 39.41 | 0.00 | 1.54 | ||
| 25Ag-400 (*) | local_1 local_2 local_3 local_4 | 65.21 40.26 7.40 64.25 | 1.24 24.64 89.95 1.39 | 33.55 35.11 2.65 34.36 | 0.019 n.c. (**) n.c. 0.022 | 1.94 n.c. n.c. 1.87 | 0.03 | 1.62 |
| 25Ag-700 (*) | local_5 | 63.88 | 0.00 | 36.12 | 0.00 | 1.77 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobs, A.; Gaulier, M.; Duval, A.; Renaudin, G. Silver Doping Mechanism in Bioceramics—From Ag+:Doped HAp to Ag°/BCP Nanocomposite. Crystals 2019, 9, 326. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9070326
Jacobs A, Gaulier M, Duval A, Renaudin G. Silver Doping Mechanism in Bioceramics—From Ag+:Doped HAp to Ag°/BCP Nanocomposite. Crystals. 2019; 9(7):326. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9070326
Chicago/Turabian StyleJacobs, Aurélie, Morgane Gaulier, Alexis Duval, and Guillaume Renaudin. 2019. "Silver Doping Mechanism in Bioceramics—From Ag+:Doped HAp to Ag°/BCP Nanocomposite" Crystals 9, no. 7: 326. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9070326