Tardigrades from Iztaccíhuatl Volcano (Trans-Mexican Volcanic Belt), with the Description of Minibiotus citlalium sp. nov. (Eutardigrada: Macrobiotidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sample Processing
2.3. Morphometrics and Morphological Nomenclature
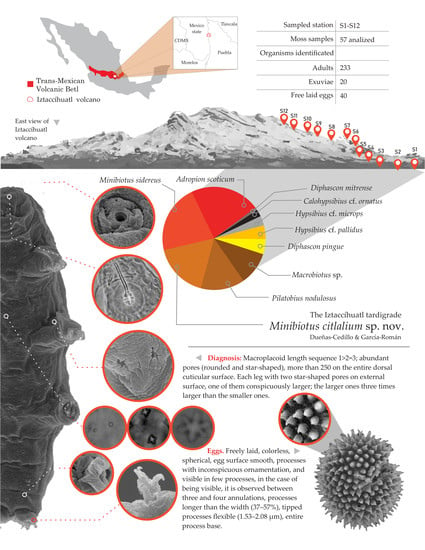
2.4. Description of Minibiotus citlalium sp. nov.
Size Effect on Morphometric Data
3. Results
Taxonomic Accounts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nelson, D.R.; Marley, N.J. The biology and ecology of lotic Tardigrada. Freshw. Biol. 2000, 44, 93–109. [Google Scholar] [CrossRef]
- Nelson, D.R.; Bartels, P.J.; Guil, N. Tardigrade Ecology. In Water Bears: The Biology of Tardigrades; Schill, R.O., Ed.; Springer Nature: Basel, Switzerland, 2018; pp. 163–210. [Google Scholar]
- Nelson, D.R. Current status of the Tardigrada: Evolution and ecology. Integr. Comp. Biol. 2002, 42, 652–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, H.A. Small-scale spatial distribution variability in terrestrial tardigrade populations. Hydrobiologia 2006, 558, 133–139. [Google Scholar] [CrossRef]
- Degma, P.; Katina, S.; Sabatovicová, L. Horizontal distribution of moisture and Tardigrada in a single moss cushion. J. Zool. Syst. Evol. Res. 2011, 49, 71–77. [Google Scholar] [CrossRef]
- Bertolani, R.; Rebecchi, L.; Giovannini, I.; Cesari, M. DNA barcoding an integrative taxonomy of Macrobiotus hufelandi C.A.S. Schultze 1834, the first tardigrade species to be described, and some related species. Zootaxa 2011, 2997, 19–36. [Google Scholar] [CrossRef]
- Bertolani, R.; Guidetti, R.; Marchioro, T.; Altiero, T.; Rebecchi, L.; Cesari, M. Phylogeny of Eutardigrada: New molecular data and their morphological support lead to the identification of new evolutionary lineages. Mol. Philogenet. Evol. 2014, 76, 110–126. [Google Scholar] [CrossRef]
- Gąsiorek, P.; Morek, W.; Stec, D.; Blagden, B.; Michalczyk, Ł. Revisiting Calohypsibiidae and Microhypsibiidae: Fractonotus Pilato, 1998 and its phylogenetic position within Isohypsibiidae (Eutardigrada: Parachela). Zoosystema 2019, 41, 71–89. [Google Scholar] [CrossRef] [Green Version]
- Mayo, S.J.; Allkin, R.; Baker, W.; Blagoderov, V.; Brake, I.; Clark, B.; Govaerts, R.; Godfray, C.; Haigh, A.; Hand, R.; et al. Alpha e-taxonomy: Responses from the systematics community to the biodiversity crisis. Kew Bull. 2008, 63, 1–16. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Diduszko, D.; Michalczyk, Ł. New records of Mexican Tardigrada. Rev. Mex. Biodivers. 2011, 82, 1324–1327. [Google Scholar] [CrossRef] [Green Version]
- Ramazzotti, G.; Maucci, W. Il Phylum Tardigrada. Terza edizione riveduta e corretta. Mem. Ist. Ital. Idrobiol. Dott. Marco Marchi 1983, 41, 1–1012. [Google Scholar]
- Challenger, A.; Soberón, J. Los ecosistemas terrestres, In Capital Natural de Mexico, Vol. I: Conocimiento Actual de la Biodiversidad; Koleff, P., Sarukhán, J.R., Eds.; Conabio: Mexico City, Mexico, 2018; pp. 87–108. [Google Scholar]
- Heinis, F. Beitrag zur Kenntnis der zentral americanischen Moosfauna. Rev. Suisse Zool. 1911, 19, 253–266. [Google Scholar] [CrossRef]
- Beasley, C.W. Some tardigrades from Mexico. Southwest. Nat. 1972, 17, 21–29. [Google Scholar] [CrossRef]
- Moreno-Talamantes, A.; Roszkowska, M.; García-Aranda, M.A.; Flores-Maldonado, J.J.; Kaczmarek, Ł. Current knowledge on Mexican tardigrades with a description of Milnesium cassandrae sp. nov. (Eutardigrada: Milnesiidae) and discussion on the taxonomic value of dorsal pseudoplates in the genus Milnesium Doyère, 1840. Zootaxa 2019, 4691, 501–524. [Google Scholar] [CrossRef] [PubMed]
- Pilato, G.; Lisi, O. Notes of some tardigrades from southern Mexico with descriptions of three new species. Zootaxa 2006, 1236, 53–68. [Google Scholar] [CrossRef]
- Schuster, R.O. Tardigrada from the Barranca del Cobre, Sinaloa y Chihuahua, Mexico. Biol. Soc. Wash. 1971, 84, 213–224. [Google Scholar]
- May, R.M. Nouveau genre et espéce de tardigrade du Mexique: Haplomacrobiotus hermosillensis. Bull. Soc. Zool. France 1948, 73, 95–97. [Google Scholar]
- Moreno-Talamantes, A.; Roszkowska, M.; Ríos-Guayasamín, P.; Flores-Maldonado, J.J.; Kaczmarek, Ł. First record of Dactylobiotus parthenogeneticus Bertolani, 1982 (Eutardigrada: Murrayidae) in Mexico. Check List 2015, 11, 1723. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Pech, W.A.; Anguas-Escalante, A.; Cutz-Pool, L.Q.; Guidetti, R. Doryphoribius chetumalensis sp. nov. (Eutardigrada: Isohypsibiidae) a new tardigrade species discovered in an unusual habitat of urban areas of Mexico. Zootaxa 2017, 4344, 347–352. [Google Scholar] [CrossRef]
- Beasley, C.W.; Kaczmarek, Ł.; Michalczyk, Ł. Doryphoribius mexicanus, a new species of Tardigrada Eutardigrada: Hypsibiidae from Mexico North America. Biol. Soc. Wash. 2008, 121, 34–40. [Google Scholar] [CrossRef]
- León-Espinosa, G.A.; Moreno-Talamantes, A.; Rodríguez-Almaraz, G.A. Ositos de agua (Tardigrada) de México: Los famosos desconocidos. Biol. Soc. 2019, 2, 61–70. Available online: https://issuu.com/biologiaysociedad/docs/biologiaysociedadn4/61 (accessed on 1 January 2020).
- Ferrari, L. Avances en el conocimiento de la Faja Volcánica Transmexicana durante la última década. Boletín de la Sociedad Geológica Mexicana 2000, 53, 84–92. [Google Scholar] [CrossRef]
- Cantellano de Rosas, E. Reconocimiento espacial de los paisajes. In Biodiversidad de la Faja Volcánica Transmexicana; Vega-Luna, I., Morrone, J.J., Espinosa, D., Eds.; Universidad Nacional Autónoma de Mexico; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2007; pp. 39–55. [Google Scholar]
- Morrone, J.J. Hacia una síntesis biogeográfica de Mexico. Rev. Mex. Biodivers. 2005, 76, 207–252. [Google Scholar] [CrossRef]
- Dastych, H. The tardigrada of Poland. Monogr Fauny Polski 1988, 16, 1–255. [Google Scholar]
- Guil, N.; Hortal, J.; Sánchez-Moreno, S.; Machordon, A. Effects of macro and micro-environmental factors on the species richness of terrestrial tardigrade assemblages in an Iberian mountain environment. Landsc. Ecol. 2009, 24, 375–390. [Google Scholar] [CrossRef]
- Nelson, D.R.; Bartels, P.J. “Smoky Bears”-Tardigrades of the Great Smoky Mountains National Park. Southwest. Nat. 2007, 6, 229–238. [Google Scholar] [CrossRef]
- Perry, E.; Miller, W.R.; Kaczmarek, Ł. Recommended abbreviations for the names of genera of the phylum Tardigrada. Zootaxa 2019, 4608, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Stec, D.; Morek, W.; Gąsiorek, P.; Michalczyk, Ł. Unmasking hidden species diversity within the Ramazzottius oberhaeuseri complex, with an integrative redescription of the nominal species for the family Ramazzottiidae (Tardigrada: Eutardigrada: Parachela). Syst. Biodivers. 2018, 16, 357–376. [Google Scholar] [CrossRef]
- Guidetti, R.; Bertolani, R. Tardigrade taxonomy: An updated check list of the taxa and a list of characters for their identification. Zootaxa 2005, 845, 1–46. [Google Scholar] [CrossRef]
- Meyer, H.A. The Terrestrial and Freshwater Tardigrada of Northeastern North America, with New Records from Maine. Notes Northeast. Nat. 2011, 4, 534–541. [Google Scholar] [CrossRef]
- Pilato, G. Analisi di nuovi caratterinello studio degli Eutardigradi. Animalia 1981, 8, 51–57. [Google Scholar]
- Pilato, G. Revision of the genus Diphascon Plate, 1889, with remarks on the subfamily Itaquasconinae (Eutardigrada, Hypsibiidae). In Biology of Tardigrades. Selected Symposia and Monographs; Bertolani, R., Ed.; Mucchi, U.Z.I.: Modena, Italy, 1987; Volume 1, pp. 337–357. [Google Scholar]
- Pilato, G.; Binda, M.G. A comparison of Diphascon (D.) alpinum Murray, 1906, D. (D.) chilenense Plate, 1889 and D. (D.) pingue Marcus, 1936 Tardigrada, and description of a new species. Zoologischer Anzeiger 1997, 236, 181–185. [Google Scholar]
- Dastych, H. A new species of the genus Macrobiotus Schultze, 1834 from Iles Kerguélen, the sub-Artic (Tardigrada). Mitteilungen Aus Dem Hamburgischen Zoologischen Mus. Und Institut 2002, 99, 11–27. [Google Scholar]
- Kaczmarek, Ł.; Michalczyk, Ł. The Macrobiotus hufelandi group (Tardigrada) revisited. Zootaxa 2017, 4363, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, Ł.; Cytan, J.; Zawierucha, K.; Diduszko, D.; Michalczyk, Ł. Tardigrades from Peru (South America), with descriptions of three new species of Parachela. Zootaxa 2014, 3790, 357–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalczyk, Ł.; Kaczmarek, Ł. The Tardigrada Register: A comprehensive online data repository for tardigrade taxonomy. J. Limnol. 2013, 72, 175–181. [Google Scholar] [CrossRef]
- Claxton, S.K. A revision of the genus Minibiotus Tardigrada: Macrobiotidae with descriptions of new species from Australia. Rec. Aust. Mus. 1998, 50, 125–160. [Google Scholar] [CrossRef] [Green Version]
- Fontoura, P.; Pilato, G. Diphascon (Diphascon) faialense sp. nov. a new species of Tardigrada Eutardigrada, Hypsibiidae from the Azores and a key to the species of the D. pingue group. Zootaxa 2007, 1589, 47–55. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Michalczyk, Ł. Redescription of Hypsibius microps Thulin, 1928 and H. pallidus Thulin, 1911 Eutardigrada: Hypsibiidae based on the type material from the Thulin collection. Zootaxa 2009, 2275, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Gąsiorek, P.; Zawierucha, K.; Stec, D.; Michalczyk, Ł. Integrative redescription of a common Arctic water bear Pilatobius recamieri Richters, 1911. Polar Biol. 2017, 40, 2239–2252. [Google Scholar] [CrossRef] [Green Version]
- Gąsiorek, P.; Stec, D.; Morek, W.; Michalczyk, Ł. An integrative redescription of Hypsibius dujardini Doyère, 1840, the nominal taxon for Hypsibioidea Tardigrada: Eutardigrada. Zootaxa 2018, 44151, 45–75. [Google Scholar] [CrossRef] [Green Version]
- Binda, M.G.; Pilato, G. Minibiotus furcatus, nuova posizione sistematica per Macrobiotus furcatus Ehrenberg, 1985 e descrizione di due nouve specie (Eutardigrada). Catania 1992, 19, 111–120. [Google Scholar]
- Pilato, G.; Binda, M.G. Two new species of Diphascon (Eutardigrada) from New South Wales. N. Z. J. Zool. 1998, 25, 171–174. [Google Scholar] [CrossRef] [Green Version]
- Pilato, G.; Binda, M.G. Three new species of Diphascon of the pingue group Eutardigrada, Hypsibiidae from Antarctica. Polar Biol. 1999, 21, 335–342. [Google Scholar] [CrossRef]
- Pilato, G.; Binda, M.G.; Qualtieri, F. Diphascon mitrense, new species of eutardigrade from Tierra del Fuego. Boll. Sedute Accad. Gioenia Sci. Nat. Catania 1999, 31, 101–105. [Google Scholar]
- Pilato, G.; Binda, M.G.; Lisi, O. Remarks on some species of tardigrades from South America with description of Minibiotus sidereus n. sp. Zootaxa 2003, 195, 1–8. [Google Scholar] [CrossRef]
- Michalczyk, Ł.; Kaczmarek, Ł. A description of the new tardigrade Macrobiotus reinhardti (Eutardigrada: Macrobiotidae, harmsworthi group) with some remarks on the oral cavity armature within the genus Macrobiotus Schultze. Zootaxa 2003, 331, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Michalczyk, Ł.; Kaczmarek, Ł. Minibiotus constellatus, new species of Tardigrada from Peru (Eutardigrada: Macrobiotidae). Genus 2003, 14, 295–305. [Google Scholar]
- Miller, W.R.; McInnes, S.J.; Bergstrøm, D.M. Tardigrades of the Australian Antarctic: Hypsibius heardensis (Eutardigrada: Hypsibiidae: Dujardini group) a new species from sub-Antarctic Heard Island. Zootaxa 2005, 1022, 57–64. [Google Scholar] [CrossRef]
- Dastych, H. Redescription and revalidation of the Sub-Antarctic tardigrade Hypsibius murrayi (Richters, 1907) based on the rediscovered type material (Tardigrada, Panarthropoda). Entomol. Heute 2018, 30, 95–115. [Google Scholar]
- Londoño, R.; Daza, A.; Lisi, O.; Quiroga, S. New species of waterbear Minibiotus pentannulatus (Tardigrada: Macrobiotidae) from Colombia. Rev. Mex. Biodivers. 2017, 88, 807–814. [Google Scholar] [CrossRef]
- Schuster, R.O.; Nelson, D.R.; Grigarick, A.A.; Christenberry, D. Systematic criteria of Eutardigrada. Trans. Am. Microsc. Soc. 1980, 99, 284–303. [Google Scholar] [CrossRef]
- Pilato, G.; Binda, G. Definition of families, subfamilies, genera and subgenera of the Eutardigrada, and keys to their identification. Zootaxa 2010, 2404, 1–54. [Google Scholar] [CrossRef] [Green Version]
- Stec, D.; Smolak, R.; Kaczmarek, Ł.; Michalczyk, Ł. An integrative description of Macrobiotus paulinae sp. nov. (Tardigrada: Eutardigrada: Macrobiotidae: Hufelandi group) from Kenya. Zootaxa 2015, 4052, 501–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilato, G.; (Catania University, Catania, Italy); Michalczyk, Ł.; (Jagiellonian University, Kraków, Poland); Londoño, R.; (Magdalena University, Santa Marta, Colombia). Personal communication, 2019.
- Lleonart, J.; Salat, J.; Torres, G.J. Removing allometric effects of body size in morphological analysis. J. Theor. Biol. 2000, 205, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Bartels, P.J.; Nelson, D.R.; Exline, R.P. Allometry and the removal of body size effects in the morphometric analysis of tardigrades. J. Zool. Syst. Evol. Res. 2011, 49, 17–25. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 2008; pp. 1–929. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 1 June 2020).
- Pilato, G. Evoluzione e nuova sistemazione degli Eutardigrada. Boll. Zool. 1969, 36, 327–345. [Google Scholar] [CrossRef] [Green Version]
- Richters, F. Beitrage zur Kenntnis der Fauna der Umgebung von Frankfurt am Main. In Beitra ge zur Kenntnis der Fauna der Umgebung von Frankfurt a. M.; 1900; pp. 21–44. Available online: https://archive.org/details/cbarchive_105587_beitrgezurkenntnisderfaunaderu1900 (accessed on 8 July 2020).
- Michalczyk, Ł.; Kaczmarek, Ł. The first record of the genus Calohypsibius Thulin, 1928 (Eutardigrada: Calohypsibiidae) from Chile (South America) with a description of a new species Calohypsibius maliki. N. Z. J. Zool. 2005, 32, 287–292. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Michalczyk, Ł.; McInnes, S.J. Annotated zoogeography of non-marine Tardigrada. Part III: North America and Greenland. Zootaxa 2016, 4203, 1–249. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Michalczyk, Ł.; McInnes, S.J. Annotated zoogeography of non-marine Tardigrada. Part II: South America. Zootaxa 2015, 3923, 1–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dastych, H. Paradiphascon manningi gen. n. sp. n., a new water-bear from South Africa, with the erecting of a new subfamily Diphasconinae (Tardigrada). Mitteilungen Aus Dem Hamburgischen Zoologischen Mus. Und Institut 1992, 89, 125–139. [Google Scholar]
- Plate, L. Beiträge zur Naturgeschichte der Tardigraden. Zoologische Jahrbücher Abteilung für Anatomie Und Ontogenie Der Tiere 1888, 3, 487–550. [Google Scholar] [CrossRef]
- Marcus, E. Tardigrada. In Das Tierreich; Schulze, F.E., Kükenthal, W., Heider, K., Eds.; Walter de Gruyter: Berlin/Leipzig, Germany, 1936; Volume 66, pp. 1–340. [Google Scholar]
- McInnes, S.J. Zoogeographic distribution of terrestrial/freshwater tardigrades from current literature. J. Nat. Hist. 1994, 28, 257–352. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Michalczyk, Ł.; McInnes, S.J. Annotated zoogeography of non-marine Tardigrada. Part I: Central America. Zootaxa 2014, 3763, 1–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrenberg, C.G. Fortgesetzte Beobachtungen über jetzt herrschende atmospharische mikroscopische, etc. mit Nachtrag und Novarum Specierum Diagnosis. Akad. Wiss. Berl. 1848, 13, 370–381. [Google Scholar]
- Rudescu, L. Tardigrada. Fauna Republicii Populare Romine. Bucuresti 1964, 4, 1–398. [Google Scholar]
- Murray, J. The Tardigrada of the Scottish Lochs. Trans. R. Soc. Edinb. 1905, 41, 677–698. [Google Scholar] [CrossRef] [Green Version]
- Li, X.C.; Liu, Y. A new subspecies of the genus Diphascon and two new records of Tardigrada (Eutardigrada: Hypsibiidae, Macrobiotidae) from China. Acta Zootaxon. Sin. 2005, 30, 309–313. [Google Scholar]
- Morgan, C.I. An Annotated Catalogue of Tardigrada in the Collections of the Royal Scottish Museum, Edinburgh; Museum Information Series; Royal Scottish: Glasgow, Scotland; Natural History: Edinburg, Scotland, 1977; Volume 5, pp. 1–29. ISSN 0307-5036. [Google Scholar]
- Ramazzotti, G. Due nuove specie di Tardigradi extra–europei. Atti Soc. Ital. Sci. Nat. Mus. Civ. Stor. Nat. Milano 1957, 96, 188–191. [Google Scholar]
- Thulin, G. Über die Phylogenie und das System der Tardigraden. Hereditas 1928, 11, 207–266. [Google Scholar] [CrossRef]
- Schultze, K.A.S. Macrobiotus Hufelandii, Animal e Crustaceorum Classe Novum, Reviviscendi Post Diuturnam Asphyxiam et Ariditatem Potens; Curths, C.A., Ed.; Apud Carolus Curths: Berlin, Germany, 1834; pp. 165–169. [Google Scholar]
- Roszkowska, M.; Stec, D.; Ciobanu, D.A.; Kaczmarek, Ł. Tardigrades from Nahuel Huapi National Park (Argentina South America) with descriptions of two new Macrobiotidae species. Zootaxa 2016, 4105, 243–260. [Google Scholar] [CrossRef]
- Michalczyk, Ł.; Kaczmarek, Ł. Minibiotus eichhorni sp. nov., a new species of eutardigrade (Eutardigrada: Macrobiotidae) from Peru. Ann. Zool. 2004, 54, 673–676. [Google Scholar]
- Stec, D.; Kristensen, R.M.; Michalczyk, Ł. An integrative description of Minibiotus ioculator sp. nov. from the Republic of South Africa with notes on Minibiotus pentannulatus Londoño et. al., 2017 (Tardigrada: Macrobiotidae). Zoologischer Anzeiger 2020, 286, 117–134. [Google Scholar] [CrossRef]
- Marcus, E. Tardigrada. In Bronn’s Klassen und Ordnungen Des Tierreichs; Akademische Verlagsgesellschaft: Leipzig, Germany, 1929; Volume 5, pp. 1–608. [Google Scholar]
- Ramazzotti, G. Il phylum Tardigrada. Memorie Dell’istituto Italiano Idrobiologia de Marchi 1962, 14, 1–595. [Google Scholar]
- Ramazzotti, G. Il Phylum Tardigrada (1° Supplemento). Memorie Dell’istituto Italiano Di Idrobiologia 1965, 19, 101–212. [Google Scholar]
- Michalczyk, Ł.; Wełnicz, W.; Frohme, M.; Kaczmarek, Ł. Redescriptions of three Milnesium Doyère, 1840 taxa (Tardigrada: Eutardigrada: Milnesiidae), including the nominal species for the genus. Zootaxa 2012, 3154, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Michalczyk, Ł.; Wełnicz, W.; Frohme, M.; Kaczmarek, Ł. Corrigenda of Zootaxa, 3154:1–20 Redescriptions of three Milnesium Doyère, 1840 taxa (Tardigrada: Eutardigrada: Milnesiidae), including the nominal species for the genus. Zootaxa 2012, 3393, 66–68. [Google Scholar] [CrossRef]
- Meyer, H.A.; Domingue, M.N. Minibiotus acadianus (Eutardigrada: Macrobiotidae), a new species of Tardigrada from southern Louisiana, U.S.A. West. N. Am. Nat. 2011, 71, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Kihm, J.; Kim, S.; McInnes, S.J.; Zawierucha, K.; Rho, H.S.; Kang, P.; Park, T.S. Integrative description of a new Dactylobiotus (Eutardigrada: Parachela) from Antarctica that reveals an intraspecific variation in tardigrade egg morphology. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Pelufo, J.R.; Rocha, A.M.; Pelufo, M.C. Species diversity and morphometrics of tardigrades in a medium–size city in the Neotropical Region: Santa Rosa (La Pampa, Argentina). Anim. Biodivers. Conserv. 2007, 30, 43–51. [Google Scholar]
- Pilato, G.; Costa, G.; Conti, E.; Binda, M.G.; Lisi, O. Morphometric analysis of some metric characters of two Macrobiotus species (Eutardigrada, Macrobiotidae). J. Limnol. 2007, 66, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Corruccini, R.S. Allometry correction in taximetrics. Syst. Zool. 1972, 21, 375–383. [Google Scholar] [CrossRef]









| Station | Sample Number | Latitude (N) | Longitude (W) | Altitude (m asl) | Vegetation Type | Substrate Type |
|---|---|---|---|---|---|---|
| S1 La Comunidad | I–IV | 19°04′24″ | 98°42′48″ | 2700 | Pinus sp., Cupressus sp., Quercus sp. | tree bark |
| S2 Barranco Palomas | V–XI | 19°04′24″ | 98°41′45″ | 3030 | Pinus sp., Cupressus sp., Quercus sp. | tree bark (V–X), soil (XI) |
| S3 Cañada Cueva del Negro | XII–XIV | 19°05′20″ | 98°40′54″ | 3278 | Abies religiosa, Cupressus sp. | tree bark (XII–XIII), soil (XIV) |
| S4 Cañada Palo Rechino 1 | XV–XIX | 19°05′30″ | 98°40′38″ | 3411 | Abies religiosa, Cupressus sp. | tree bark (XV–XVI), soil (XVII–XIX) |
| S5 Cañada El Paraje * | XX *–XXII | 19°05′14″ | 98°40′03″ | 3498 | Abies religiosa, Cupressus sp. | tree bark (XX), soil (XXI–XXII) |
| S6 Cañada Palo Rechino 2 | XXIII–XXVIII | 19°04′52″ | 98°39′40″ | 3613 | Abies religiosa, Cupressus sp. | tree bark (XXVII–XXVIII), soil (XXIII–XXVI) |
| S7 Paso de Cortés | XXIX–XXXII | 19°05′06″ | 98°38′49″ | 3700 | Pinus hartwegii | tree bark (XXX, XXXII), soil (XXIX, XXXI) |
| S8 Subestación Eléctrica | XXXIII–XXXVII | 19°06′13″ | 98°38′43″ | 4007 | Pinus hartwegii | tree bark (XXXIII–XXXV), soil (XXXVI–XXXVII) |
| S9 Refugio Altzomoni | XXXVIII–XLII | 19°07′31″ | 98°39′11″ | 3957 | Pinus hartwegii | soil (XXXVIII–XXXIX), rock (XL–XLII) |
| S10 Parador La Joya | XLIII–XLVII | 19°08′10″ | 98°38′56″ | 4126 | Pinus hartwegii | soil (XLVII), rock (XLIII–XLVI) |
| S11 Primer Portillo | XLVIII–LII | 19°08′31″ | 98°38′30″ | 4235 | alpine scrub | soil (XLVIII), rock XLIX–LII) |
| S12 Segundo Portillo | LIII–LVII | 19°08′42″ | 98°38′19″ | 4500 | alpine scrub | soil (LIII–LIV), rock (LV–LVII) |
| CHARACTER | N | RANGE | MEAN | SD | HOLOTYPE | ||||
|---|---|---|---|---|---|---|---|---|---|
| µm | pt | µm | pt | µm | pt | µm | pt | ||
| Body length | 15 | 142–250 | 507–925 | 195 | 746 | 37 | 121 | 142.4 | 507 |
| Buccal tube | |||||||||
| Buccal tube length | 16 | 20.2–28.7 | 26.1 | - | 2.4 | - | 28.1 | - | |
| Stylet support insertion point | 16 | 12.1–17.0 | 57.7–61.5 | 15.6 | 59.9 | 1.5 | 1.0 | 17.0 | 60.2 |
| Buccal tube external width | 16 | 1.5–2.4 | 5.7–8.6 | 2.0 | 7.5 | 0.3 | 0.7 | 2.0 | 7.7 |
| Buccal tube internal width | 16 | 0.7–1.5 | 2.4–5.3 | 1.1 | 4.1 | 0.3 | 0.8 | 1.0 | 3.7 |
| Ventral lamina length | 15 | 9.2–13.4 | 44.6–49.1 | 12.1 | 46.3 | 1.3 | 1.3 | 12.9 | 45.9 |
| Placoid length | |||||||||
| Macroplacoid 1 | 15 | 1.2–2.8 | 5.6–9.9 | 2.0 | 7.7 | 0.4 | 1.3 | 2.5 | 8.9 |
| Macroplacoid 2 | 15 | 1.1–2.3 | 5.1–8.4 | 1.7 | 6.6 | 0.3 | 1.0 | 2.0 | 7.1 |
| Macroplacoid 3 | 15 | 1.3–2.3 | 5.1–8.0 | 1.7 | 6.6 | 0.3 | 0.8 | 2.0 | 7.1 |
| Microplacoid | 15 | 0.5–1.1 | 1.9–3.8 | 0.7 | 2.8 | 0.2 | 0.6 | 0.9 | 3.0 |
| Macroplacoid row | 15 | 4.7–7.6 | 21.5–27.2 | 6.3 | 24.6 | 0.8 | 1.7 | 7.6 | 27.2 |
| Placoid row | 15 | 5.9–9.0 | 26.5–33.4 | 7.6 | 29.3 | 0.8 | 1.9 | 9.0 | 32.0 |
| Claw 1 height | |||||||||
| External primary branch | 15 | 3.1–5.8 | 11.8–20.7 | 4.6 | 17.4 | 0.9 | 2.8 | 5.8 | 20.7 |
| External secondary branch | 15 | 2.0–4.4 | 7.4–16.3 | 3.3 | 3.1 | 0.9 | 2.8 | 4.3 | 15.3 |
| Internal primary branch | 15 | 3.5–5.8 | 12.4–21.6 | 4.8 | 8.1 | 0.8 | 2.2 | 5.7 | 20.1 |
| Internal secondary branch | 15 | 2.9–4.9 | 10.4–18.2 | 3.9 | 14.8 | 0.7 | 2.1 | 4.2 | 15.1 |
| Claw 2 height | |||||||||
| External primary branch | 13 | 3.8–6.2 | 18.6–21.1 | 5.2 | 20.1.9 | 0.7 | 1.2 | 6.2 | 22.1 |
| External secondary branch | 12 | 2.5–5.3 | 12.1–19.6 | 4.0 | 15.3 | 0.9 | 2.4 | 4.7 | 16.9 |
| Internal primary branch | 13 | 3.3–6.0 | 14.8–21.5 | 4.8 | 18.7 | 0.9 | 2.3 | 6.0 | 21.5 |
| Internal secondary branch | 12 | 2.3–5.0 | 10.3–17.9 | 3.9 | 14.8 | 0.8 | 2.1 | 4.5 | 16.0 |
| Claw 3 height | |||||||||
| External primary branch | 14 | 3.8–5.7 | 17.0–22.1 | 4.9 | 18.8 | 0.5 | 1.5 | 4.8 | 17.0 |
| External secondary branch | 13 | 2.9–4.6 | 13.0–17.4 | 3.7 | 14.5 | 0.5 | 1.2 | 4.1 | 14.2 |
| Internal primary branch | 14 | 3.6–5.9 | 16.7–22.9 | 4.9 | 19.1 | 0.7 | 1.7 | 5.0 | 17.8 |
| Internal secondary branch | 13 | 2.7–5.3 | 12.7–18.6 | 3.9 | 15.2 | 0.8 | 1.8 | 4.5 | 16.0 |
| Claw 4 length | |||||||||
| Anterior primary branch | 13 | 3.3–6.9 | 14.0–25.8 | 5.6 | 21.3 | 1.1 | 3.0 | 5.5 | 19.5 |
| Anterior secondary branch | 12 | 2.5–5.3 | 10.7–20.5 | 4.4 | 16.6 | 1.1 | 3.3 | 4.6 | 16.5 |
| Posterior primary branch | 11 | 3.1–7.0 | 13.2–26.3 | 5.7 | 21.57 | 1.3 | 3.8 | - | - |
| Posterior secondary branch | 11 | 2.1–5.9 | 10.4–21.8 | 4.0 | 15.2 | 1.1 | 3.2 | - | - |
| CHARACTER | N | RANGE | MEAN | SD |
|---|---|---|---|---|
| Number of pores on cuticle | ||||
| Multi-lobated pores | ||||
| 1. up to legs I | 3 | 6 | 6 | |
| 2. up to legs II | 3 | 5–8 | 6.67 | 1.11 |
| 3. up to legs III | 3 | 8–14 | 10.67 | 2.22 |
| 4. up to legs IV | 3 | 8–9 | 8.67 | 0.44 |
| Star-shaped pores | ||||
| 1. up to legs I | 3 | 6–10 | 7.67 | 1.56 |
| 2. up to legs II | 3 | 5–8 | 6.33 | 1.11 |
| 3. up to legs III | 3 | 2–4 | 3.67 | 1.11 |
| 4. up to legs IV | 3 | 7–10 | 8.33 | 1.11 |
| CHARACTER | N | RANGE | MEAN | SD |
|---|---|---|---|---|
| Diameter of egg without processes | 3 | 48.8–60.8 | 54.6 | 6.0 |
| Diameter of egg with processes | 3 | 57.4–66.0 | 60.9 | 4.5 |
| Process height | 9 | 3.6–5.1 | 4.4 | 0.5 |
| Process base width | 8 | 1.8–2.6 | 2.1 | 0.3 |
| Process base/height ratio | 8 | 37–57% | 48% | 6% |
| Distance between processes | 9 | 1.2–2.6 | 1.8 | 0.4 |
| Number of processes on the egg circumference | 2 | 28–33 | 30.5 | 3.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dueñas-Cedillo, A.; Martínez-Méndez, E.; García-Román, J.; Armendáriz-Toledano, F.; Ruiz, E.A. Tardigrades from Iztaccíhuatl Volcano (Trans-Mexican Volcanic Belt), with the Description of Minibiotus citlalium sp. nov. (Eutardigrada: Macrobiotidae). Diversity 2020, 12, 271. https://0-doi-org.brum.beds.ac.uk/10.3390/d12070271
Dueñas-Cedillo A, Martínez-Méndez E, García-Román J, Armendáriz-Toledano F, Ruiz EA. Tardigrades from Iztaccíhuatl Volcano (Trans-Mexican Volcanic Belt), with the Description of Minibiotus citlalium sp. nov. (Eutardigrada: Macrobiotidae). Diversity. 2020; 12(7):271. https://0-doi-org.brum.beds.ac.uk/10.3390/d12070271
Chicago/Turabian StyleDueñas-Cedillo, Alba, Evelyn Martínez-Méndez, Jazmín García-Román, Francisco Armendáriz-Toledano, and Enrico Alejandro Ruiz. 2020. "Tardigrades from Iztaccíhuatl Volcano (Trans-Mexican Volcanic Belt), with the Description of Minibiotus citlalium sp. nov. (Eutardigrada: Macrobiotidae)" Diversity 12, no. 7: 271. https://0-doi-org.brum.beds.ac.uk/10.3390/d12070271