Salinity Affects Freshwater Invertebrate Traits and Litter Decomposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Outdoor Mesocosms
2.2. Leaf Litter Decomposition and Associated Invertebrates
2.3. Invertebrate Communities of the Mesocosms
2.4. Invertebrate Life Cycle Traits
2.5. Data Analysis
3. Results
3.1. Abiotic Variables
3.2. Leaf Litter Decomposition and Associated Invertebrates
3.3. Invertebrate Coomunities in the Mesocosms
4. Discussion
4.1. Leaf Litter Decomposition and Associated Invertebrates
4.2. Invertebrates in the Mesocosms
4.3. Invertebrate Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., Eds.; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; in press. [Google Scholar]
- Werner, A.D.; Bakker, M.; Post, V.E.A.; Vandenbohede, B.; Lu, C.; Ataie-Ashtiani, A.; Simmons, C.T.; Barry, D.A. Seawater intrusion processes, investigation and management: Recent advances and future challenges. Adv. Water Resour. 2012, 51, 3–26. [Google Scholar] [CrossRef]
- Cañedo-Argüelles, M.; Kefford, B.; Schäfer, R. Salt in freshwaters: Causes, effects and prospects-introduction to the theme issue. Phil. Trans. R. Soc. B. 2018, 374, 20180002. [Google Scholar] [CrossRef]
- Iglesias, M.C.-A. A review of recent advances and future challenges in freshwater salinization. Limnetica 2020, 39, 185–211. [Google Scholar] [CrossRef]
- Kaushal, S.S.; Likens, G.E.; Pace, M.L.; Reimer, J.E.; Maas, C.M.; Galella, J.G.; Utz, R.M.; Duan, S.; Kryger, J.R.; Yaculak, A.M.; et al. Freshwater salinization syndrome: From emerging global problem to managing risks. Biogeochemistry 2021, 154, 255–292. [Google Scholar] [CrossRef]
- Venâncio, C.; Ribeiro, R.; Lopes, I. Seawater intrusion: An appraisal of taxa at most risk and safe salinity levels. Biol. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, G.; Arivoli, S.; Venkatesan, P. Effect of salinity on the predatory performance of Diplonychus rusticus (Fabricius). J. Environ. Biol. 2008, 29, 287–290. [Google Scholar] [PubMed]
- Venâncio, C.; Anselmo, E.; Soares, A.; Lopes, I. Does increased salinity influence the competitive outcome of two producer species? Environ. Sci. Pollut. Res. 2017, 24, 5888–5897. [Google Scholar] [CrossRef]
- Venâncio, C.; Ribeiro, R.; Lopes, I. Active emigration from climate change-caused seawater intrusion into freshwater habitats. Environ. Pollut. 2020, 258, 113805. [Google Scholar] [CrossRef]
- Gessner, M.O.; Chauvet, E. A case for using litter breakdown to assess functional stream integrity. Ecol. Appl. 2002, 12, 498–510. [Google Scholar] [CrossRef]
- Roache, M.C.; Bailey, P.C.; Boon, P.I. Effects of salinity on the decay of the freshwater macrophyte, Triglochin procerum. Aquat. Bot. 2006, 84, 45–52. [Google Scholar] [CrossRef]
- Berger, E.; Frör, O.; Schäfer, R.B. Salinity impacts on river ecosystem processes: A critical mini-review. Phil. Trans. R. Soc. B 2018, 374, 20180010. [Google Scholar] [CrossRef] [Green Version]
- Júnior, E.S.A.; Martínez, A.; Gonçalves, A.L.; Canhoto, C. Combined effects of freshwater salinization and leaf traits on litter decomposition. Hydrobiologia 2020, 847, 3427–3435. [Google Scholar] [CrossRef]
- Graça, M.A.S. The Role of Invertebrates on Leaf Litter Decomposition in Streams—A Review. Int. Rev. Hydrobiol. 2001, 86, 383–393. [Google Scholar] [CrossRef]
- Kefford, B.J.; Dalton, A.; Palmer, C.G.; Nugegoda, D. The salinity tolerance of eggs and hatchlings of selected aquatic macroinvertebrates in south-east Australia and South Africa. Hydrobiologia 2004, 517, 179–192. [Google Scholar] [CrossRef]
- Venâncio, C.; Castro, B.B.; Ribeiro, R.; Antunes, S.C.; Abrantes, N.; Soares, A.M.V.M.; Lopes, I. Sensitivity of freshwater species under single and multigenerational exposure to seawater intrusion. Phil. Trans. R. Soc. B 2018, 374, 20180252. [Google Scholar] [CrossRef] [Green Version]
- Verberk, W.C.E.P.; Siepel, H.; Esselink, H. Life-history strategies in freshwater macroinvertebrates. Freshw. Biol. 2008, 53, 1722–1738. [Google Scholar] [CrossRef]
- Venâncio, C.A.R. Salinization Effects on Coastal Terrestrial and Freshwater Ecosystems. Ph.D. Thesis, Universidade de Aveiro, Aveiro, Portugal, 2017; pp. 139–166. [Google Scholar]
- Pérez, J.; Basaguren, A.; López-Rojo, N.; Tonin, A.M.; Correa-Araneda, F.; Boyero, L. The Role of Key Plant Species on Litter Decomposition in Streams: Alder as Experimental Model. In The Ecology of Plant Litter Decomposition in Stream Ecosystems; Swan, C.M., Boyero, L., Canhoto, C., Eds.; Springer: Berlin, Germany, 2021; pp. 143–161. [Google Scholar] [CrossRef]
- Tachet, H.; Richoux, P.; Bournaud, M.; Usseglio-Polatera, P. Invertébrés D’eau Douce–Systématique, Biologie, Ecologie; CNRS Éditions: Paris, France, 2000. [Google Scholar]
- Gutiérrez-Cánovas, C.; Sánchez-Fernández, D.; Cañedo-Argüelles, M.; Millán, A.; Velasco, J.; Acosta, R.; Fortuño, P.; Otero, N.; Soler, A.; Bonada, N. Do all roads lead to Rome? Exploring community trajectories in response to anthropogenic salinization and dilution of rivers. Phil. Trans. R. Soc. B 2019, 374, 20180009. [Google Scholar] [CrossRef] [Green Version]
- Zar, J.H. Biostatistical Analysis; Prentice Hall, Inc.: Upper Saddle River, NJ, USA, 1996; ISBN 0-13-086398-X. [Google Scholar]
- Sauer, F.G.; Bundschuh, M.; Zubrod, J.P.; Schäfer, R.B.; Thompson, K.; Kefford, B.J. Effects of salinity on leaf breakdown: Dryland salinity versus salinity from a coalmine. Aquat. Toxicol. 2016, 177, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Werba, J.A.; Stucy, A.L.; Peralta, A.L.; McCoy, M.W. Effects of diversity and coalescence of species assemblages on ecosystem function at the margins of an environmental shift. Peer J. 2020, 8, e8608. [Google Scholar] [CrossRef] [Green Version]
- Hart, B.T.; Bailey, P.; Edwards, R.; Hortle, K.; James, K.; McMahon, A.; Meredith, C.; Swadling, K. Effects of salinity on river, stream and wetland ecosystems in Victoria, Australia. Water Res. 1990, 24, 1103–1117. [Google Scholar] [CrossRef]
- Piscart, C.; Moreteau, J.-C.; Beisel, J.-N. Biodiversity and structure of macroinvertebrate communities along a small permanent salinity gradient (Meurthe River, France). Hydrobiologia 2005, 551, 227–236. [Google Scholar] [CrossRef]
- Herbert, E.R.; Boon, P.; Burgin, A.J.; Neubauer, S.C.; Franklin, R.B.; Ardón, M.; Hopfensperger, K.N.; Lamers, L.P.M.; Gell, P. A global perspective on wetland salinization: Ecological consequences of a growing threat to freshwater wetlands. Ecosphere 2015, 6, 206. [Google Scholar] [CrossRef]
- Rutherford, J.C.; Kefford, B.J. Effects of Salinity on Stream Ecosystems: Improving Models for Macroinvertebrates. In CSIRO Land and Water Technical Report 22/05; CSIRO Land and Water: Canberra, Australia, 2005. [Google Scholar]
- Kefford, B.J.; Nugegoda, D. No evidence for a critical salinity threshold for growth and reproduction in the freshwater snail Physa acuta. Environ. Pollut. 2005, 134, 377–383. [Google Scholar] [CrossRef]
- Kefford, B.J.; Papas, P.J.; Nugegoda, D. Relative salinity tolerance of macroinvertebrates from the Barwon River, Victoria, Australia. Mar. Freshw. Res. 2003, 54, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Dunlop, J.E.; Horrigan, N.; McGregor, G.; Kefford, B.J.; Choy, S.; Prasad, R. Effect of spatial variation on salinity tolerance of macroinvertebrates in Eastern Australia and implications for ecosystem protection trigger values. Environ. Pollut. 2008, 151, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Hassell, K.L.; Kefford, B.J.; Nugegoda, D. Sub-lethal and chronic salinity tolerances of three freshwater insects: Cloeon sp. and Centroptilum sp. (Ephemeroptera: Baetidae) and Chironomus sp. (Diptera: Chironomidae). J. Exp. Biol. 2006, 209, 4024–4032. [Google Scholar] [CrossRef] [Green Version]
- Hilsenhoff, W.L. Diversity and Classification of Insects and Collembola. In Ecology and Classification of North American Freshwater Invertebrates, 2nd ed.; Thorp, J.H., Covich, A.P., Eds.; Academic Press: Cambridge, MA, USA, 2001; pp. 661–731. [Google Scholar] [CrossRef]
- Kefford, B.J.; Fields, E.J.; Clay, C.; Nugegoda, D. Salinity tolerance of riverine microinvertebrates from the southern Murray-Darling Basin. Mar. Freshw. Res. 2007, 58, 1019–1031. [Google Scholar] [CrossRef] [Green Version]



| Treatment | Electrical Conductivity (mS cm−1) | Dissolved Oxygen (mg L−1) | Temperature (°C) | pH | Nitrate (mg N-NO3 L−1) | Ammonia (mg N-NH3 L−1) | Orthophosphate (mg PO4 3− L−1) | Sulfate (mg SO4 2− L−1) |
|---|---|---|---|---|---|---|---|---|
| Before | n = 2 | n = 2 | n = 2 | n = 2 | n = 1 | n = 1 | n = 1 | n = 1 |
| All mesocosms | 0.27 ± 0.01 (0.26–0.27) | 10.9 ± 0.6 (9.6–11.9) | 14.9 ± 2.4 (12.3–17.3) | 8.0 ± 0.1 (7.8–8.2) | 2.9 ± 0.9 (1.8–4.2) | 0.19 ± 0.10 (0.07–0.34) | 0.05 ± 0.03 (0.01–0.08) | 10 ± 3 (7–16) |
| During | n = 6 | n = 4 | n = 5 | n = 6 | n = 6 | n = 6 | n = 6 | n = 5 |
| Control (0.28 mS cm−1) | 0.26 ± 0.05 (0.18–0.29) | 12.0 ± 1.7 (10.1–14.2) | 8.0 ± 1.6 (6.0–10.0) | 8.2 ± 0.6 (7.8–9.4) | 4.3 ± 0.7 (2.0–6.9) | 0.14 ± 0.12 (0.02–0.33) | 0.07 ± 0.06 (0.01–0.17) | 15 ± 12 (7–36) |
| 2.0 mS cm−1 | 1.86 ± 0.31 (1.26–2.05) | 17.5 ± 2.5 (13.9–19.2) | 8.1 ± 1.6 (6.1–10.0) | 8.3 ± 0.5 (7.8–9.2) | 3.8 ± 0.8 (3.3–4.8) | 0.36 ± 0.36 (0.00–0.48) | 0.09 ± 0.06 (0.05–0.16) | 104 ± 54 (56–184) |
| 3.3 mS cm−1 | 3.05 ± 0.53 (2.10–3.34) | 14.4 ± 0.5 (13.9–15.0) | no data | 8.3 ± 0.4 (7.9–9.0) | 3.6 ± 0.4 (3.0–4.0) | 0.25 ± 0.20 (0.01–0.60) | 0.08 ± 0.06 (0.04–0.15) | 132 ± 57 (74–220) |
| 5.5 mS cm−1 | 5.10 ± 0.66 (3.85–5.52) | 14.5 ± 1.1 (12.8–15.3) | 8.5 ± 1.6 (6.6–10.3) | 8.3 ± 0.4 (7.9–9.2) | 3.7 ± 1.1 (2.2–5.2) | 0.36 ± 0.33 (0.01–0.95) | 0.09 ± 0.04 (0.05–0.17) | 233 ± 170 (112–504) |
| 9.3 mS cm−1 | 8.58 ± 1.02 (6.61–9.22) | 14.2 ± 1.8 (12.7–16.3) | 8.3 ± 1.7 (6.3–10.1) | 8.4 ± 0.4 (7.9–9.0) | 4.1 ± 1.6 (2.9–6.9) | 0.70 ± 0.70 (0.02–1.86) | 0.08 ± 0.05 (0.03–0.15) | 341 ± 136 (188–520) |
| 15.3 mS cm−1 | 14.01 ± 1.62 (11.00–15.12) | 14.8 ± 0.9 (13.6–15.7) | 8.2 ± 1.7 (6.1–10.0) | 8.5 ± 0.4 (8.0–9.0) | 4.3 ± 1.7 (2.0–6.9) | 2.12 ± 0.93 (0.29–2.75) | 0.11 ± 0.06 (0.08–0.21) | 466 ± 241 (282–828) |
| Leaf Litter Decomposition | |||||
| All data (n = 6) | 2.0 mS cm−1 | 3.3 mS cm−1 | 5.5 mS cm−1 | 9.3 mS cm−1 | 15.3 mS cm−1 |
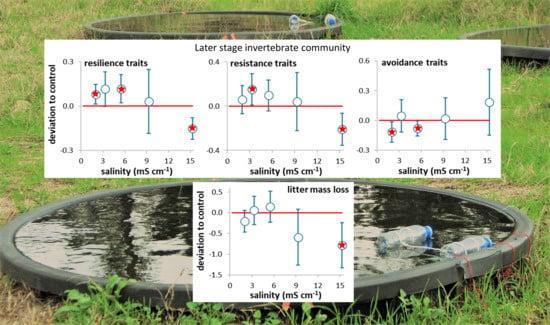
| Mass loss | −0.21 ± 0.26 | 0.06 ± 0.34 | 0.15 ± 0.37 | −0.59 ± 0.67 | −0.78 ± 0.55 |
| Invertebrate abundance | 0.64 ± 0.80 | 0.14 ± 0.41 | 0.85 ± 1.28 | 0.33 ± 0.88 | 0.17 ± 0.38 |
| Invertebrate richness | 0.22 ± 0.55 | −0.13 ± 0.32 | −0.06 ± 0.31 | −0.16 ± 0.13 | −0.33 ± 0.21 |
| (a) Resilience traits | −0.09 ± 0.18 | −0.01 ± 0.29 | 0.33 ± 0.14 | 0.19 ± 0.26 | 0.22 ± 0.38 |
| (b) Resistance traits | −0.34 ± 0.47 | 0.02 ± 0.57 | 0.74 ± 0.54 | 0.50 ± 0.80 | 0.69 ± 0.85 |
| (c) Avoidance traits | 0.39 ± 0.21 | −0.19 ± 0.41 | −0.27 ± 0.33 | −0.23 ± 0.35 | −0.40 ± 0.49 |
| Day 63 (n = 3) | 2.0 mS cm−1 | 3.3 mS cm−1 | 5.5 mS cm−1 | 9.3 mS cm−1 | 15.3 mS cm−1 |
| (a) Resilience traits | 0.05 ± 0.16 | 0.18 ± 0.34 | 0.44 ± 0.02 | 0.40 ± 0.13 | 0.47 ± 0.11 |
| (b) Resistance traits | 0.04 ± 0.17 | 0.40 ± 0.60 | 1.17 ± 0.35 | 1.13 ± 0.49 | 1.17 ± 0.33 |
| (c) Avoidance traits | 0.56 ± 0.10 | −0.40 ± 0.58 | −0.54 ± 0.12 | −0.46 ± 0.36 | −0.67 ± 0.65 |
| Invertebrate Communities in the Mesocosms | |||||
| All sampling days (n = 6) | 2.0 mS cm−1 | 3.3 mS cm−1 | 5.5 mS cm−1 | 9.3 mS cm−1 | 15.3 mS cm−1 |
| Invertebrate abundance | −0.17 ± 0.35 | −0.34 ± 0.24 | −0.25 ± 0.20 | 0.01 ± 0.55 | −0.15 ± 0.87 |
| Invertebrate richness | −0.14 ± 0.26 | −0.28 ± 0.20 | −0.08 ± 0.24 | −0.07 ± 0.26 | 0.07 ± 0.24 |
| (a) Resilience traits | 0.06 ± 0.04 | 0.13 ± 0.07 | 0.08 ± 0.08 | 0.04 ± 0.10 | −0.09 ± 0.14 |
| (b) Resistance traits | 0.06 ± 0.07 | 0.16 ± 0.10 | 0.05 ± 0.14 | 0.05 ± 0.13 | −0.14 ± 0.18 |
| (c) Avoidance traits | 0.01 ± 0.13 | 0.11 ± 0.19 | −0.05 ± 0.12 | 0.02 ± 0.10 | 0.06 ± 0.23 |
| Days 21–65 (n = 3) | 2.0 mS cm−1 | 3.3 mS cm−1 | 5.5 mS cm−1 | 9.3 mS cm−1 | 15.3 mS cm−1 |
| (a) Resilience traits | 0.08 ± 0.08 | 0.11 ± 0.12 | 0.12 ± 0.10 | 0.03 ± 0.22 | −0.15 ± 0.07 |
| (b) Resistance traits | 0.06 ± 0.13 | 0.15 ± 0.14 | 0.10 ± 0.14 | 0.04 ± 0.26 | −0.21 ± 0.14 |
| (c) Avoidance traits | −0.12 ± 0.10 | 0.04 ± 0.16 | −0.08 ± 0.08 | 0.02 ± 0.21 | 0.18 ± 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abelho, M.; Ribeiro, R.; Moreira-Santos, M. Salinity Affects Freshwater Invertebrate Traits and Litter Decomposition. Diversity 2021, 13, 599. https://0-doi-org.brum.beds.ac.uk/10.3390/d13110599
Abelho M, Ribeiro R, Moreira-Santos M. Salinity Affects Freshwater Invertebrate Traits and Litter Decomposition. Diversity. 2021; 13(11):599. https://0-doi-org.brum.beds.ac.uk/10.3390/d13110599
Chicago/Turabian StyleAbelho, Manuela, Rui Ribeiro, and Matilde Moreira-Santos. 2021. "Salinity Affects Freshwater Invertebrate Traits and Litter Decomposition" Diversity 13, no. 11: 599. https://0-doi-org.brum.beds.ac.uk/10.3390/d13110599