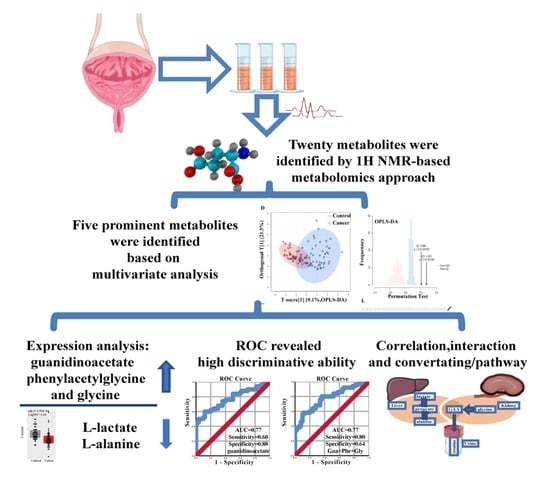
Novel Metabolic Signatures of Prostate Cancer Revealed by 1H-NMR Metabolomics of Urine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Samples Selection and Ethics Statement
2.2. Sample Preparation and 1H-NMR Based Metabolomics Analysis
2.3. Data Modelling and Statistical Analysis
2.4. Identification of Relevant Metabolites
2.5. Acquisition of the Pathways and Biological Processes Corresponding to Metabolites
2.6. Statistics
3. Results
3.1. Metabolites in Urine Samples of PC
3.2. Identification of Important Metabolites and the Metabolic Changes
3.3. Acquisition of the Most Prominent Metabolites, Correlation Analysis, and ROC Analysis
3.4. Subgroup Analysis
3.5. Analysis of the Metabolite Interaction Networks and Corresponding Pathways
4. Discussion
4.1. The Location and Expression of Metabolites in PC
4.2. Potential Biomarkers of PC
4.3. Metabolite Interactions and Pathways Potentially Involved in PC
4.4. The Major Findings of the Present Study
- The metabolites guanidinoacetate, phenylacetylglycine, and glycine were significantly upregulated in urine samples of PC. On the contrary, l-alanine and l-lactate were significantly downregulated. Furthermore, the majority of them were positively correlated. Especially strong correlations were seen between guanidinoacetate, phenylacetylglycine and glycine.
- Guanidinoacetate, phenylacetylglycine, and glycine urine levels were significantly different between PC patients stratified for low GS (≤6) and high GS (≥7).
- Using the network module, we comprehensively described the potential interaction between the most prominent metabolites. ROC analyses of prominent metabolites revealed a reasonably high diagnostic accuracy of guanidinoacetate, phenylacetylglycine, and glycine.
- Pathway enrichment analysis indicated “Glycine, Serine, and Threonine metabolism” as the most importantly altered pathway. Those results provide evidence for the metabolites, and associated pathway potentially playing an essential role in PC.
- Here, we reported for the first time that guanidinoacetate, and phenylacetylglycine could be promising novel urine biomarkers for PC.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the receiver operating characteristic (ROC) curve |
| CI | Confidence Interval |
| DRE | Digital rectal examination |
| FC | Fold change |
| GAA | Guanidinoacetate |
| GC/MS | Gas chromatography-mass spectrometry |
| GS | Gleason score |
| HMDB | Human Metabolome Database |
| NMR | Nuclear magnetic resonance |
| OPLS-DA | Orthogonal Partial Least Squares discriminant analysis |
| PBS | Phosphate buffer solution |
| PC | Prostate cancer |
| PCA | Principal component analysis |
| PLS-DA | Partial Least Squares discriminant analysis |
| PSA | Prostate specific antigen |
| ROC | Receiver operating characteristic curve |
| STITCH | Search tool for interactions of chemicals |
| TSP | Trimethylsilylpropionic acid-d4 sodium salt |
| TURP | Transurethral resection of the prostate |
| VIP | Variable importance in projection |
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velonas, V.M.; Woo, H.H.; dos Remedios, C.G.; Assinder, S.J. Current status of biomarkers for prostate cancer. Int. J. Mol. Sci. 2013, 14, 11034–11060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickers, A.J.; Cronin, A.M.; Aus, G.; Pihl, C.G.; Becker, C.; Pettersson, K.; Scardino, P.T.; Hugosson, J.; Lilja, H. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: Data from the European Randomized Study of Prostate Cancer Screening in Göteborg, Sweden. BMC Med. 2008, 6, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Link, R.E.; Shariat, S.F.; Nguyen, C.V.; Farr, A.; Weinberg, A.D.; Morton, R.A.; Richardson, B.; Bernard, D.; Slawin, K.M. Variation in prostate specific antigen results from 2 different assay platforms: Clinical impact on 2304 patients undergoing prostate cancer screening. J. Urol. 2004, 171, 2234–2238. [Google Scholar] [CrossRef] [PubMed]
- McDunn, J.E.; Li, Z.; Adam, K.P.; Neri, B.P.; Wolfert, R.L.; Milburn, M.V.; Lotan, Y.; Wheeler, T.M. Metabolomic signatures of aggressive prostate cancer. Prostate 2013, 73, 1547–1560. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.J.; Schirra, H.J.; Lavin, M.F.; Gardiner, R.A. Metabolomics: A novel approach to early and noninvasive prostate cancer detection. Korean J. Urol. 2011, 52, 79–89. [Google Scholar] [CrossRef]
- Djavan, B.; Zlotta, A.; Remzi, M.; Ghawidel, K.; Basharkhah, A.; Schulman, C.C.; Marberger, M. Optimal predictors of prostate cancer on repeat prostate biopsy: A prospective study of 1,051 men. J. Urol. 2000, 163, 1144–1149. [Google Scholar] [CrossRef]
- Rigau, M.; Olivan, M.; Garcia, M.; Sequeiros, T.; Montes, M.; Colás, E.; Llauradó, M.; Planas, J.; Torres, I.; Morote, J.; et al. The present and future of prostate cancer urine biomarkers. Int. J. Mol. Sci. 2013, 14, 12620–12649. [Google Scholar] [CrossRef] [Green Version]
- Wilkosz, J.; Bryś, M.; Różański, W. Urine markers and prostate cancer. Cent. Eur. J. Urol. 2011, 64, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Watson, D.G.; Wang, L.; Abbas, M.; Murdoch, L.; Bashford, L.; Ahmad, I.; Lam, N.Y.; Ng, A.C.; Leung, H.Y. Application of Holistic Liquid Chromatography-High Resolution Mass Spectrometry Based Urinary Metabolomics for Prostate Cancer Detection and Biomarker Discovery. PLoS ONE 2013, 8, e65880. [Google Scholar] [CrossRef] [Green Version]
- Chistiakov, D.A.; Myasoedova, V.A.; Grechko, A.V.; Melnichenko, A.A.; Orekhov, A.N. New biomarkers for diagnosis and prognosis of localized prostate cancer. Semin. Cancer Biol. 2018, 52, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Gordetsky, J.; Epstein, J. Grading of prostatic adenocarcinoma: Current state and prognostic implications. Diagn. Pathol. 2016, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, R.K.; Satkunavisam, R.; Chin, J.L.; Izawa, J.; Trachtenberg, J.; Rendon, R.; Bell, D.; Singal, R.; Sherman, C.; Sugar, L.; et al. Next-generation prostate cancer risk calculator for primary care physicians. Journal de l’Association des Urologues du Canada 2018, 12, e64–e70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Meo, A.; Bartlett, J.; Cheng, Y.; Pasic, M.D.; Yousef, G.M. Liquid biopsy: A step forward towards precision medicine in urologic malignancies. Mol. Cancer 2017, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, A.; Blanchet, L.; Buydens, L.M.; Wijmenga, S.S. NMR and pattern recognition methods in metabolomics: From data acquisition to biomarker discovery: A review. Anal. Chim. Acta 2012, 750, 82–97. [Google Scholar] [CrossRef]
- Srivastava, S.; Roy, R.; Singh, S.; Kumar, P.; Dalela, D.; Sankhwar, S.N.; Goel, A.; Sonkar, A.A. Taurine—A possible fingerprint biomarker in non-muscle invasive bladder cancer: A pilot study by 1H NMR spectroscopy. Cancer Biomark. 2010, 6, 11–20. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, S.; Liu, L.; Nagana Gowda, G.A.; Bonney, P.; Stewart, J.; Knapp, D.W.; Raftery, D. NMR-based metabolomics study of canine bladder cancer. Biochim. Biophys. Acta 2012, 1822, 1807–1814. [Google Scholar] [CrossRef] [Green Version]
- Bertini, I.; Cacciatore, S.; Jensen, B.V.; Schou, J.V.; Johansen, J.S.; Kruhøffer, M.; Luchinat, C.; Nielsen, D.L.; Turano, P. Metabolomic NMR fingerprinting to identify and predict survival of patients with metastatic colorectal cancer. Cancer Res. 2012, 72, 356–364. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Liao, G.Q.; Wen, X.F.; Chen, W.H.; Cheng, S.; Stolzenburg, J.U.; Ganzer, R.; Neuhaus, J. Nuclear magnetic resonance spectroscopy as a new approach for improvement of early diagnosis and risk stratification of prostate cancer. J. Zhejiang Univ. Sci. B 2017, 18, 921–933. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Deng, Y.; Ye, J.; Zhuo, Y.; Liu, Z.; Liang, Y.; Zhang, H.; Zhu, X.; Luo, Y.; Feng, Y.; et al. Aberrant Expression of Citrate Synthase is Linked to Disease Progression and Clinical Outcome in Prostate Cancer. Cancer Manag. Res. 2020, 12, 6149–6163. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Cancer Res. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Cancer Res. 2016, 55, 14.10.11–14.10.91. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an Optimized Workflow for Global Metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, E.A. Ropls: PCA, PLS (-DA) and OPLS (-DA) for Multivariate Analysis and Feature Selection of Omics Data. 2016. Available online: https://0-scholar-google-com.brum.beds.ac.uk/ (accessed on 12 August 2020).
- Rohart, F.; Gautier, B.; Singh, A.; KA, L.C. mixOmics: An R package for ’omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [Green Version]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. Biosyst. 2015, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2012, 8, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, M.; von Mering, C.; Campillos, M.; Jensen, L.J.; Bork, P. STITCH: Interaction networks of chemicals and proteins. Nucleic Acids Res. 2008, 36, D684–D688. [Google Scholar] [CrossRef]
- Jewison, T.; Su, Y.; Disfany, F.M.; Liang, Y.; Knox, C.; Maciejewski, A.; Poelzer, J.; Huynh, J.; Zhou, Y.; Arndt, D.; et al. SMPDB 2.0: Big improvements to the Small Molecule Pathway Database. Nucleic Acids Res. 2014, 42, D478–D484. [Google Scholar] [CrossRef] [Green Version]
- Frolkis, A.; Knox, C.; Lim, E.; Jewison, T.; Law, V.; Hau, D.D.; Liu, P.; Gautam, B.; Ly, S.; Guo, A.C.; et al. SMPDB: The Small Molecule Pathway Database. Nucleic Acids Res. 2010, 38, D480–D487. [Google Scholar] [CrossRef] [Green Version]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The human urine metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef] [Green Version]
- Salek, R.M.; Maguire, M.L.; Bentley, E.; Rubtsov, D.V.; Hough, T.; Cheeseman, M.; Nunez, D.; Sweatman, B.C.; Haselden, J.N.; Cox, R.D.; et al. A metabolomic comparison of urinary changes in type 2 diabetes in mouse, rat, and human. Physiol. Genom. 2007, 29, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zijlstra, C.; Stoorvogel, W. Prostasomes as a source of diagnostic biomarkers for prostate cancer. J. Clin. Investig. 2016, 126, 1144–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öman, T.; Tessem, M.B.; Bathen, T.F.; Bertilsson, H.; Angelsen, A.; Hedenström, M.; Andreassen, T. Identification of metabolites from 2D (1)H-(13)C HSQC NMR using peak correlation plots. BMC Bioinform. 2014, 15, 413. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Ahmed, S. Emerging field of metabolomics: Big promise for cancer biomarker identification and drug discovery. J. Pharm. Biomed. Anal. 2015, 107, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Ryan, D.; Brennan, L.; Tenori, L.; Luchinat, C.; Gao, X.; Zeri, A.C.; Gowda, G.A.; et al. Recommendations and Standardization of Biomarker Quantification Using NMR-Based Metabolomics with Particular Focus on Urinary Analysis. J. Proteome Res. 2016, 15, 360–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. Available online: https://hmdb.ca/ (accessed on 19 January 2021). [CrossRef] [PubMed]
- MacKinnon, N.; Ge, W.; Han, P.; Siddiqui, J.; Wei, J.T.; Raghunathan, T.; Chinnaiyan, A.M.; Rajendiran, T.M.; Ramamoorthy, A.J.N.P.C. NMR-Based Metabolomic Profiling of Urine: Evaluation for Application in Prostate Cancer Detection. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Adamko, D.; Rowe, B.H.; Marrie, T.; Sykes, B.D.J.M. Variation of Metabolites in Normal Human Urine. Metabolomics 2007, 3, 439–451. [Google Scholar]
- Lima, A.R.; Bastos Mde, L.; Carvalho, M.; Guedes de Pinho, P. Biomarker Discovery in Human Prostate Cancer: An Update in Metabolomics Studies. Transl. Oncol. 2016, 9, 357–370. [Google Scholar] [CrossRef] [Green Version]
- Giskeødegård, G.F.; Bertilsson, H.; Selnæs, K.M.; Wright, A.J.; Bathen, T.F.; Viset, T.; Halgunset, J.; Angelsen, A.; Gribbestad, I.S.; Tessem, M.B. Spermine and citrate as metabolic biomarkers for assessing prostate cancer aggressiveness. PLoS ONE 2013, 8, e62375. [Google Scholar] [CrossRef] [Green Version]
- Dinges, S.S.; Hohm, A.; Vandergrift, L.A.; Nowak, J.; Habbel, P.; Kaltashov, I.A.; Cheng, L.L. Cancer metabolomic markers in urine: Evidence, techniques and recommendations. Nat. Rev. Urol. 2019, 16, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gupta, A.; Mandhani, A.; Sankhwar, S.N. Metabolomics-derived prostate cancer biomarkers: Fact or fiction? J. Proteome Res. 2015, 14, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, Y.; Higashiyama, M.; Gochi, A.; Akaike, M.; Ishikawa, T.; Miura, T.; Saruki, N.; Bando, E.; Kimura, H.; Imamura, F.; et al. Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS ONE 2011, 6, e24143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, M.; Zhang, C.H.; Kosugi, C.; Matsumoto, I. Gas chromatography-mass spectrometric studies of canine urinary metabolism. J. Vet. Med Sci. 1995, 57, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Rambla, C.; Puchades-Carrasco, L.; García-Flores, M.; Rubio-Briones, J.; López-Guerrero, J.A.; Pineda-Lucena, A. Non-invasive urinary metabolomic profiling discriminates prostate cancer from benign prostatic hyperplasia. Metabolomics 2017, 13, 52. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, H.; Xie, L.-x.; Li, X.; Zhang, A.-H.J.R.A. High-throughput metabolomics enables biomarker discovery in prostate cancer. RSC Adv. 2017, 7, 2587–2593. [Google Scholar] [CrossRef] [Green Version]
- Serkova, N.J.; Gamito, E.J.; Jones, R.H.; O’Donnell, C.; Brown, J.L.; Green, S.; Sullivan, H.; Hedlund, T.; Crawford, E.D. The metabolites citrate, myo-inositol, and spermine are potential age-independent markers of prostate cancer in human expressed prostatic secretions. Prostate 2008, 68, 620–628. [Google Scholar] [CrossRef]
- Sreekumar, A.; Poisson, L.M.; Rajendiran, T.M.; Khan, A.P.; Cao, Q.; Yu, J.; Laxman, B.; Mehra, R.; Lonigro, R.J.; Li, Y.; et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 2009, 457, 910–914. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Liu, T.; Ma, C.; Xue, R.; Deng, C.; Zeng, H.; Shen, X. GC/MS-based metabolomic approach to validate the role of urinary sarcosine and target biomarkers for human prostate cancer by microwave-assisted derivatization. Anal. Bioanal. Chem. 2011, 401, 635–646. [Google Scholar] [CrossRef]
- Lees, H.J.; Swann, J.R.; Poucher, S.; Holmes, E.; Wilson, I.D.; Nicholson, J.K. Obesity and Cage Environment Modulate Metabolism in the Zucker Rat: A Multiple Biological Matrix Approach to Characterizing Metabolic Phenomena. J. Proteome Res. 2019, 18, 2160–2174. [Google Scholar] [CrossRef]
- DeFeo, E.M.; Wu, C.L.; McDougal, W.S.; Cheng, L.L. A decade in prostate cancer: From NMR to metabolomics. Nat. Rev. Urol. 2011, 8, 301–311. [Google Scholar] [CrossRef]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review. Oxidative Med. Cell. Longev. 2017, 2017, 1716701. [Google Scholar] [CrossRef]
- Shibano, K.; Kawamura, S.; Hakamada, R.; Kawamura, Y. The relationship between changes in serum glycine and alanine concentrations in non-essential amino acid and milk production in the transition period in dairy cows. J. Veter- Med Sci. 2005, 67, 191–193. [Google Scholar] [CrossRef] [Green Version]
- Patra, S.K.; Patra, A.; Zhao, H.; Dahiya, R. DNA methyltransferase and demethylase in human prostate cancer. Mol. Carcinog. 2002, 33, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Carreira, S.; Sandhu, S.; Miranda, S.; Mossop, H.; Perez-Lopez, R.; Nava Rodrigues, D.; Robinson, D.; Omlin, A.; Tunariu, N.; et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N. Engl. J. Med. 2015, 373, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Amelio, I.; Cutruzzolá, F.; Antonov, A.; Agostini, M.; Melino, G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 2014, 39, 191–198. [Google Scholar] [CrossRef]
- Duan, L.M.; Liu, J.Y.; Yu, C.W.; Fan, J.X.; Li, T.; Yang, J.X.; Zheng, Y.B.; Liu, F.C.; He, Z.T.; Yuan, H.L.; et al. PLCε knockdown prevents serine/glycine metabolism and proliferation of prostate cancer by suppressing YAP. Am. J. Cancer Res. 2020, 10, 196–210. [Google Scholar]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Kim, H.; Kalchman, I.; Santiago-Jiménez, M.; Lehrer, J.; Guo, J.; Hermann, G.; Yamoah, K.; Alshalalfa, M.; Huang, H.C.; Ross, A.E.; et al. Transcriptome evaluation of the relation between body mass index and prostate cancer outcomes. Cancer 2017, 123, 2240–2247. [Google Scholar] [CrossRef] [Green Version]
- Delaney, J.; Neville, W.A.; Swain, A.; Miles, A.; Leonard, M.S.; Waterfield, C.J. Phenylacetylglycine, a putative biomarker of phospholipidosis: Its origins and relevance to phospholipid accumulation using amiodarone treated rats as a model. Biomarkers 2004, 9, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Park, J.C.; Shin, M.J.; Lee, H.; Oh, J.; Ryu, D.H.; Hwang, G.S.; Chung, J.H. ¹H nuclear magnetic resonance based metabolic urinary profiling of patients with ischemic heart failure. Clinical Biochem. 2011, 44, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Jung, Y.; Bang, E.J.; Cho, S.I.; Jang, Y.J.; Kwak, J.M.; Ryu, D.H.; Park, S.; Hwang, G.S. Noninvasive diagnosis and evaluation of curative surgery for gastric cancer by using NMR-based metabolomic profiling. Ann. Surg. Oncol. 2014, 21 (Suppl. 4), S736–S742. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU guidelines on prostate cancer. part 1: Screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014, 65, 124–137. [Google Scholar] [CrossRef] [PubMed]







| Characteristics | Control Group (n = 50) | PC Group (n = 50) | Significance | ||
|---|---|---|---|---|---|
| Mean (SD) | Group Size | Mean (SD) | Group Size | p-Value | |
| Age (years) | 63.30 (9.61) | 50 | 70.00 (8.98) | 50 | <0.0001 |
| Prostate volume (mL) | 26.24 (8.77) | 24 | 39.77(19.00) | 50 | 0.0169 |
| PSA (≤ 10 ng/mL) | 1.56 (0.89) | 50 | 6.69 (1.96) | 14 | |
| PSA (10.1–20 ng/mL) | NA | 0 | 14.01 (2.08) | 14 | |
| PSA (> 20 ng/mL) | NA | 0 | 89.82 (86.28) | 22 | |
| GS (pre) 6 | NA | NA | NA | 13 | |
| GS (pre) ≥7 | NA | NA | NA | 34 | |
| GS (post) 6 | NA | NA | NA | 6 | |
| GS (post) ≥7 | NA | NA | NA | 35 | |
| Treatment: | 50 | ||||
| Radical operation | 41 | ||||
| Seed implantation | 5 | ||||
| Endocrine | 2 | ||||
| Chemotherapy | 1 | ||||
| TURP | 1 | ||||
| Key | Metabolites | HMDB ID | Moieties | Chemical Shifts a | VIP |
|---|---|---|---|---|---|
| 1 | L-lactate | HMDB0000190 | αCH, βCH3 | 1.33 (d,J = 6.6Hz),4.13 (q,J = 4.8Hz) | 1.43 |
| 2 | L-alanine | HMDB0000161 | βCH3 | 1.48 (d, J = 7.2Hz) | 1.76 |
| 3 | acetate | HMDB0000042 | CH3 | 1.92 (s) | 1.45 |
| 5 | succinate | HMDB0000254 | CH2 | 2.41 (s) | 0.06 |
| 6 | citrate | HMDB0000094 | half CH2, half CH2 | 2.54 (d,J = 16.2 Hz), 2.70 (d, J = 15.6 Hz) | 0.42 |
| 7 | dimethylglycine | HMDB0000092 | N-CH3, CH2 | 2.92 (s), 3.72 (s) | 1.06 |
| 8 | formate | HMDB0000142 | CH | 8.46 (s) | 0.99 |
| 11 | dimethylamine | HMDB0000087 | CH3 | 2.73 (s) | 0.82 |
| 12 | methylguanidine | HMDB0001522 | CH3 | 2.85 (s) | 0.17 |
| 13 | trimethylamine | HMDB0000906 | CH3 | 2.88 (s) | 0.89 |
| 14 | creatinine | HMDB0000562 | CH3, CH2 | 3.04 (s), 4.06 (s) | 0.45 |
| 15 | taurine | HMDB0000251 | S-CH2, N-CH2 | 3.27 (t), 3.42 (t) | 0.29 |
| 16 | betaine | HMDB0000043 | N(CH3)3, CH2 | 3.27 (s), 3.90 (s) | 0.09 |
| 17 | guanidinoacetate | HMDB0000128 | CH2 | 3.80 (s) | 1.94 |
| 18 | hippurate | HMDB0000714 | CH2, CH, CH, CH | 3.97 (d,J = 6Hz), 7.55 (t,J = 7.8Hz), 7.64 (t,J = 7.8Hz), 7.84 (d,J = 7.2Hz) | 0.02 |
| 19 | N-methylnicotinamide | HMDB0003152 | 2-CH, 4-CH, 6-CH, 5-CH, CH3 | 9.29 (s), 8.97 (d,J = 6Hz), 8.91 (dt), 8.19 (m), 4.48 (s) | 0.55 |
| 20 | 2-Hydroxyisobutyrate | HMDB0000729 | CH3 | 1.36 (s) | 0.36 |
| 21 | glycine | HMDB0000123 | CH2 | 3.57 (s) | 1.36 |
| 22 | fumaric acid | HMDB0000134 | CH | 6.56 (s) | 0.32 |
| 28 | Phenylacetylglycine | HMDB0000821 | CH2, CH, CH | 3.68 (s), 7.37 (m), 7.43 (m) | 1.59 |
| Metabolites | Samples (Methods) | Reference | Ethnos | |
|---|---|---|---|---|
| Up-Regulated | Down-Regulated | |||
| BCAA, glutamate; pseudouridine | Glycine @, dimethylglycine @, fumarate, 4-imidazole-acetate | Urine (1H-NMR) | Pérez-Rambla et al. [46] | Spanish |
| glycocholic acid, hippurate, chenodeoxycholic acid | 5-Hydroxy-l-tryptophan, taurocholic acid | Urine (FPLC/MS) | Liang, et al. [47] | Chinese (Northern of China) |
| citrate, Myo-inositol, spermine | EPS (1H-NMR) | Serkova et al. [48] | American | |
| sarcosine | Urine/PT/Plasma (GC-MS) | Sreekumr et al. [49] | American | |
| propenoic acid, dihyroxybutanoic acid xylonic acid | pyrimidine, creatinine, purine, glucopyranoside, xylopyranoseand, ribofuranoside | Urine (GC-MS) | Wu et al. [50] | Chinese (Southern of China) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Zhang, C.; Cheng, S.; Li, G.; Griebel, J.; Neuhaus, J. Novel Metabolic Signatures of Prostate Cancer Revealed by 1H-NMR Metabolomics of Urine. Diagnostics 2021, 11, 149. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11020149
Yang B, Zhang C, Cheng S, Li G, Griebel J, Neuhaus J. Novel Metabolic Signatures of Prostate Cancer Revealed by 1H-NMR Metabolomics of Urine. Diagnostics. 2021; 11(2):149. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11020149
Chicago/Turabian StyleYang, Bo, Chuan Zhang, Sheng Cheng, Gonghui Li, Jan Griebel, and Jochen Neuhaus. 2021. "Novel Metabolic Signatures of Prostate Cancer Revealed by 1H-NMR Metabolomics of Urine" Diagnostics 11, no. 2: 149. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11020149