Biomarkers in the Light of the Etiopathology of IC/BPS
Abstract
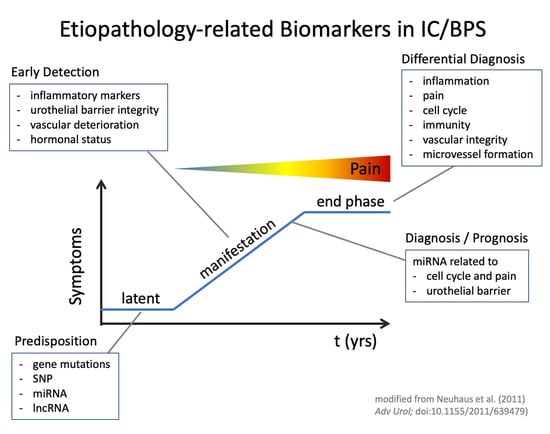
:1. Introduction
2. Materials and Methods
3. Results
3.1. Genetic Factors—Potential Drivers of IC/BPS
3.2. Gene Expression, Networks and Signaling Pathways
3.3. Occult Urinary Tract Infections and the Microbiome
3.4. Virus Infections in IC/BPS?
3.5. Mast Cells and Lymphocytes as Biomarkers
3.6. Platelet Activating Factor (PAF)
3.7. The Role and Regulation of Vascular Endothelial Growth Factor (VEGF)
3.8. Alterations of the Urothelial Barrier—A Hallmark of IC/BPS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Davis, N.F.; Brady, C.M.; Creagh, T. Interstitial cystitis/painful bladder syndrome: Epidemiology, pathophysiology and evidence-based treatment options. Eur. J. Obs. Gynecol. Reprod. Biol. 2014, 175, 30–37. [Google Scholar] [CrossRef]
- Homma, Y.; Ueda, T.; Tomoe, H.; Lin, A.T.; Kuo, H.C.; Lee, M.H.; Lee, J.G.; Kim, D.Y.; Lee, K.S.; Interstitial, C.G.C. Clinical guidelines for interstitial cystitis and hypersensitive bladder syndrome. Int. J. Urol. 2009, 16, 597–615. [Google Scholar] [CrossRef]
- Park, J.M. Is interstitial cystitis an underdiagnosed problem in children? A diagnostic and therapeutic dilemma. Urology 2001, 57 (Suppl. 1), 30–31. [Google Scholar] [CrossRef]
- van de Merwe, J.P.; Nordling, J.; Bouchelouche, P.; Bouchelouche, K.; Cervigni, M.; Daha, L.K.; Elneil, S.; Fall, M.; Hohlbrugger, G.; Irwin, P.; et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: An ESSIC proposal. Eur. Urol. 2008, 53, 60–67. [Google Scholar] [CrossRef]
- Homma, Y.; Akiyama, Y.; Tomoe, H.; Furuta, A.; Ueda, T.; Maeda, D.; Lin, A.T.; Kuo, H.C.; Lee, M.H.; Oh, S.J.; et al. Clinical guidelines for interstitial cystitis/bladder pain syndrome. Int. J. Urol. 2020, 27, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Maeda, D.; Akiyama, Y.; Morikawa, T.; Kunita, A.; Ota, Y.; Katoh, H.; Niimi, A.; Nomiya, A.; Ishikawa, S.; Goto, A.; et al. Hunner-Type (Classic) Interstitial Cystitis: A Distinct Inflammatory Disorder Characterized by Pancystitis, with Frequent Expansion of Clonal B-Cells and Epithelial Denudation. PLoS ONE 2015, 10, e0143316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, J.J.; Chen, Y.K.; Lin, H.C. Comorbidities of bladder pain syndrome/interstitial cystitis: A population-based study. BJU Int. 2012, 110 (11 Pt C), E903–E909. [Google Scholar] [CrossRef] [Green Version]
- Majima, T.; Sassa, N. Organ cross-sensitization mechanisms in chronic diseases related to the genitourinary tract. J. Smooth Muscle Res. 2021, 57, 49–52. [Google Scholar] [CrossRef]
- Kujala, M.M.; Tammela, T.L.; Pöyhönen, A.; Forsell, T.; Pasanen, S.; Paananen, I.; Horte, A.; Leppilahti, M.; Sairanen, J. Prevalence of autoimmune disorders among bladder pain syndrome patients’ relatives. Scand. J. Urol. 2021, 55, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.D.; Huang, C.C.; Lin, H.C.; Kao, L.T. Bladder pain syndrome/interstitial cystitis is associated with asthma: A case-control study. Neurourol. Urodyn 2018, 37, 1773–1778. [Google Scholar] [CrossRef]
- Lee, C.K.; Tsai, C.P.; Liao, T.L.; Huang, W.N.; Chen, Y.H.; Lin, C.H.; Chen, Y.M. Overactive bladder and bladder pain syndrome/interstitial cystitis in primary Sjögren’s syndrome patients: A nationwide population-based study. PLoS ONE 2019, 14, e0225455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, E.; Silva, R.; Romão, V.C.; Neves, M.; Garcia, R.; Oliveira, S.; Brites, J.; Ramos, F.O.; Canhão, H.; Palma Dos Reis, J.; et al. Overactive bladder symptom bother and health-related quality of life in patients with systemic lupus erythematosus and primary Sjögren syndrome. Lupus 2019, 28, 27–33. [Google Scholar] [CrossRef]
- Barton, J.C.; Bertoli, L.F.; Barton, J.C.; Acton, R.T. Fibromyalgia in 300 adult index patients with primary immunodeficiency. Clin. Exp. Rheumatol. 2017, 35 (Suppl. 105), 68–73. [Google Scholar] [PubMed]
- Wen, J.Y.; Lo, T.S.; Chuang, Y.C.; Ho, C.H.; Long, C.Y.; Law, K.S.; Tong, Y.C.; Wu, M.P. Risks of interstitial cystitis among patients with systemic lupus erythematosus: A population-based cohort study. Int. J. Urol. 2019, 26, 897–902. [Google Scholar] [CrossRef]
- Yueh, H.Z.; Yang, M.H.; Huang, J.Y.; Wei, J.C. Risk of Autoimmune Diseases in Patients With Interstitial Cystitis/Bladder Pain Syndrome: A Nationwide Population-Based Study in Taiwan. Front. Med. 2021, 8, 8747098. [Google Scholar] [CrossRef]
- Tirlapur, S.A.; Kuhrt, K.; Chaliha, C.; Ball, E.; Meads, C.; Khan, K.S. The ‘evil twin syndrome’ in chronic pelvic pain: A systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int. J. Surg. 2013, 11, 233–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Merwe, J.P.; Yamada, T.; Sakamoto, Y. Systemic aspects of interstitial cystitis, immunology and linkage with autoimmune disorders. Int. J. Urol. 2003, 10, S35–S38. [Google Scholar] [CrossRef]
- Chung, S.D.; Liu, S.P.; Lin, C.C.; Li, H.C.; Lin, H.C. Bladder pain syndrome/interstitial cystitis is associated with hyperthyroidism. PLoS ONE 2013, 8, e72284. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.W.; Jackson, T.L.; Langenberg, P.; Meyers, D.J.; Xu, J. Prevalence of interstitial cystitis in first-degree relatives of patients with interstitial cystitis. Urology 2004, 63, 17–21. [Google Scholar] [CrossRef]
- Altman, D.; Lundholm, C.; Milsom, I.; Peeker, R.; Fall, M.; Iliadou, A.N.; Pedersen, N.L. The genetic and environmental contribution to the occurrence of bladder pain syndrome: An empirical approach in a nationwide population sample. Eur. Urol. 2011, 59, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Allen-Brady, K.; Norton, P.A.; Cannon-Albright, L. Risk of associated conditions in relatives of subjects with interstitial cystitis. Female Pelvic Med. Reconstr. Surg. 2015, 21, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Allen-Brady, K.; Rowe, K.; Cessna, M.; Lenherr, S.; Norton, P. Significant Linkage Evidence for Interstitial Cystitis/Painful Bladder Syndrome on Chromosome 3. J. Urol. 2018, 199, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Cassão, V.D.; Reis, S.T.; Pimenta, R.; Lucon, M.; Leite, K.R.M.; Srougi, M.; Bruschini, H. Single nucleotide polymorphism analysis in interstitial cystitis/painful bladder syndrome. PLoS ONE 2019, 14, e0215201. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Li, S.; Sun, F.; Wang, G.; Wei, D.; Yang, T.; Gu, S. Bioinformatics analysis of the Hub genes and key pathways of interstitial cystitis pathogenesis. Neurourol. Urodyn. 2020, 39, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, J.; Zhao, X.; Liang, C.; Wang, Y.; Sun, L.; Jiang, Z.; Zhang, Z.; Yang, R.; Chen, J.; et al. MicroRNA expression signatures of bladder cancer revealed by deep sequencing. PLoS ONE 2011, 6, e18286. [Google Scholar] [CrossRef] [Green Version]
- Arai, T.; Fuse, M.; Goto, Y.; Kaga, K.; Kurozumi, A.; Yamada, Y.; Sugawara, S.; Okato, A.; Ichikawa, T.; Yamanishi, T.; et al. Molecular pathogenesis of interstitial cystitis based on microRNA expression signature: miR-320 family-regulated molecular pathways and targets. J. Hum. Genet. 2018, 63, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Gheinani, A.H.; Burkhard, F.C.; Monastyrskaya, K. Deciphering microRNA code in pain and inflammation: Lessons from bladder pain syndrome. Cell Mol. Life Sci. 2013, 70, 3773–3789. [Google Scholar] [CrossRef] [Green Version]
- Monastyrskaya, K.; Sánchez-Freire, V.; Hashemi Gheinani, A.; Klumpp, D.J.; Babiychuk, E.B.; Draeger, A.; Burkhard, F.C. miR-199a-5p regulates urothelial permeability and may play a role in bladder pain syndrome. Am. J. Pathol. 2013, 182, 431–448. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Huang, L.; Luo, Q.; Tu, Q.; Liu, J.; Yu, R.; Huang, J.; Chen, T.; Yin, Y.; Cao, J. Absence of Toll-like receptor 7 protects mice against Pseudomonas aeruginosa pneumonia. Int. Immunopharmacol. 2021, 96, 107739. [Google Scholar] [CrossRef]
- Xagorari, A.; Chlichlia, K. Toll-like receptors and viruses: Induction of innate antiviral immune responses. Open Microbiol. J. 2008, 2, 49–59. [Google Scholar] [CrossRef]
- Clancy, R.M.; Markham, A.J.; Buyon, J.P. Endosomal Toll-like receptors in clinically overt and silent autoimmunity. Immunol. Rev. 2016, 269, 76–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Chen, Y.; Liu, R.; Chen, B.; Liu, C.; Xing, J. Long noncoding RNA (MEG3) in urinal exosomes functions as a biomarker for the diagnosis of Hunner-type interstitial cystitis (HIC). J. Cell Biochem. 2020, 121, 1227–1237. [Google Scholar] [CrossRef]
- Ichihara, K.; Aizawa, N.; Akiyama, Y.; Kamei, J.; Masumori, N.; Andersson, K.E.; Homma, Y.; Igawa, Y. Toll-like receptor 7 is overexpressed in the bladder of Hunner-type interstitial cystitis, and its activation in the mouse bladder can induce cystitis and bladder pain. Pain 2017, 158, 1538–1545. [Google Scholar] [CrossRef]
- Joseph, M.; Enting, D. Immune Responses in Bladder Cancer-Role of Immune Cell Populations, Prognostic Factors and Therapeutic Implications. Front. Oncol. 2019, 9, 1270. [Google Scholar] [CrossRef] [Green Version]
- Aydogan, T.B.; Gurpinar, O.; Eser, O.K.; Mathyk, B.A.; Ergen, A. A new look at the etiology of interstitial cystitis/bladder pain syndrome: Extraordinary cultivations. Int. Urol. Nephrol. 2019, 51, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Price, T.K.; Dune, T.; Hilt, E.E.; Thomas-White, K.J.; Kliethermes, S.; Brincat, C.; Brubaker, L.; Wolfe, A.J.; Mueller, E.R.; Schreckenberger, P.C. The Clinical Urine Culture: Enhanced Techniques Improve Detection of Clinically Relevant Microorganisms. J. Clin. Microbiol. 2016, 54, 1216–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meriwether, K.V.; Lei, Z.; Singh, R.; Gaskins, J.; Hobson, D.T.G.; Jala, V. The Vaginal and Urinary Microbiomes in Premenopausal Women With Interstitial Cystitis/Bladder Pain Syndrome as Compared to Unaffected Controls: A Pilot Cross-Sectional Study. Front. Cell Infect Microbiol. 2019, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhide, A.; Tailor, V.; Khullar, V. Interstitial cystitis/bladder pain syndrome and recurrent urinary tract infection and the potential role of the urinary microbiome. Post Reprod. Health 2020, 26, 87–90. [Google Scholar] [CrossRef]
- Jhang, J.F.; Hsu, Y.H.; Peng, C.W.; Jiang, Y.H.; Ho, H.C.; Kuo, H.C. Epstein-Barr Virus as a Potential Etiology of Persistent Bladder Inflammation in Human Interstitial Cystitis/Bladder Pain Syndrome. J. Urol. 2018, 200, 590–596. [Google Scholar] [CrossRef]
- Salamonowicz-Bodzioch, M.; Frączkiewicz, J.; Czyżewski, K.; Zając-Spychała, O.; Gorczyńska, E.; Panasiuk, A.; Ussowicz, M.; Kałwak, K.; Szmit, Z.; Wróbel, G.; et al. Prospective analysis of BKV hemorrhagic cystitis in children and adolescents undergoing hematopoietic cell transplantation. Ann. Hematol. 2021, 100, 1283–1293. [Google Scholar] [CrossRef]
- Van der Aa, F.; Beckley, I.; de Ridder, D. Polyomavirus BK--a potential new therapeutic target for painful bladder syndrome/interstitial cystitis. Med. Hypotheses 2014, 83, 317–320. [Google Scholar] [CrossRef]
- Oberbach, A.; Schlichting, N.; Feder, S.; Lehmann, S.; Kullnick, Y.; Buschmann, T.; Blumert, C.; Horn, F.; Neuhaus, J.; Neujahr, R.; et al. New insights into valve-related intramural and intracellular bacterial diversity in infective endocarditis. PLoS ONE 2017, 12, e0175569. [Google Scholar] [CrossRef]
- Mumm, J.N.; Osterman, A.; Ruzicka, M.; Stihl, C.; Vilsmaier, T.; Munker, D.; Khatamzas, E.; Giessen-Jung, C.; Stief, C.; Staehler, M.; et al. Urinary Frequency as a Possibly Overlooked Symptom in COVID-19 Patients: Does SARS-CoV-2 Cause Viral Cystitis. Eur. Urol. 2020, 78, 624–628. [Google Scholar] [CrossRef]
- Lamb, L.E.; Dhar, N.; Timar, R.; Wills, M.; Dhar, S.; Chancellor, M.B. COVID-19 inflammation results in urine cytokine elevation and causes COVID-19 associated cystitis (CAC). Med. Hypotheses 2020, 145, 110375. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Barclay, D.; Zamora, R.; Yoshimura, N.; Peters, K.; Vodovotz, Y.; Chancellor, M. Urine cytokines suggest an inflammatory response in the overactive bladder: A pilot study. Int. Urol. Nephrol. 2010, 42, 629–635. [Google Scholar] [CrossRef]
- Gamper, M.; Regauer, S.; Welter, J.; Eberhard, J.; Viereck, V. Are mast cells still good biomarkers for bladder pain syndrome/interstitial cystitis. J. Urol. 2015, 193, 1994–2000. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Maeda, D.; Morikawa, T.; Niimi, A.; Nomiya, A.; Yamada, Y.; Igawa, Y.; Goto, A.; Fukayama, M.; Homma, Y. Digital quantitative analysis of mast cell infiltration in interstitial cystitis. Neurourol. Urodyn 2018, 37, 650–657. [Google Scholar] [CrossRef] [Green Version]
- Bahler, D.W.; Swerdlow, S.H. Clonal salivary gland infiltrates associated with myoepithelial sialadenitis (Sjögren’s syndrome) begin as nonmalignant antigen-selected expansions. Blood 1998, 91, 1864–1872. [Google Scholar] [CrossRef] [Green Version]
- Doorenspleet, M.E.; Klarenbeek, P.L.; de Hair, M.J.H.; van Schaik, B.D.C.; Esveldt, R.E.E.; van Kampen, A.H.C.; Gerlag, D.M.; Musters, A.; Baas, F.; Tak, P.P.; et al. Rheumatoid arthritis synovial tissue harbours dominant B-cell and plasma-cell clones associated with autoreactivity. Ann. Rheum. Dis. 2014, 73, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.I.; Medeiros, J.A. Role of Helicobacter pylori in gastric mucosa-associated lymphoid tissue lymphomas. World J. Gastroenterol. 2014, 20, 684–698. [Google Scholar] [CrossRef]
- Pich, D.; Mrozek-Gorska, P.; Bouvet, M.; Sugimoto, A.; Akidil, E.; Grundhoff, A.; Hamperl, S.; Ling, P.D.; Hammerschmidt, W. First Days in the Life of Naive Human B Lymphocytes Infected with Epstein-Barr Virus. mBio 2019, 10, e01723-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamper, M.; Viereck, V.; Geissbuhler, V.; Eberhard, J.; Binder, J.; Moll, C.; Rehrauer, H.; Moser, R. Gene expression profile of bladder tissue of patients with ulcerative interstitial cystitis. BMC Genom. 2009, 10, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadas, P.; Gold, M.; Perelman, B.; Liss, G.M.; Lack, G.; Blyth, T.; Simons, F.E.; Simons, K.J.; Cass, D.; Yeung, J. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N. Engl. J. Med. 2008, 358, 28–35. [Google Scholar] [CrossRef]
- Kispert, S.E.; Marentette, J.; Campian, E.C.; Isbell, T.S.; Kuenzel, H.; McHowat, J. Cigarette smoke-induced urothelial cell damage: Potential role of platelet-activating factor. Physiol. Rep. 2017, 5, e13177. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, S.J.; Seo, K.W.; Bae, J.U.; Park, S.Y.; Kim, E.K.; Bae, S.S.; Kim, J.H.; Kim, C.D. PAF enhances MMP-2 production in rat aortic VSMCs via a β-arrestin2-dependent ERK signaling pathway. J. Lipid Res. 2013, 54, 2678–2686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottino, P.; Bazan, H.E. Corneal stimulation of MMP-1, -9 and uPA by platelet-activating factor is mediated by cyclooxygenase-2 metabolites. Curr. Eye Res. 2001, 23, 77–85. [Google Scholar] [CrossRef]
- Xu, L.F.; Teng, X.; Guo, J.; Sun, M. Protective effect of intestinal trefoil factor on injury of intestinal epithelial tight junction induced by platelet activating factor. Inflammation 2012, 35, 308–315. [Google Scholar] [CrossRef]
- Al-Zahrani, A.A.; Gajewski, J.B. Long-term efficacy and tolerability of pentosan polysulphate sodium in the treatment of bladder pain syndrome. Can. Urol. Assoc. J. 2011, 5, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Jhang, J.-F.; Birder, L.A.; Jiang, Y.-H.; Hsu, Y.-H.; Ho, H.-C.; Kuo, H.-C. Dysregulation of bladder corticotropin-releasing hormone receptor in the pathogenesis of human interstitial cystitis/bladder pain syndrome. Sci. Rep. 2019, 9, 19169. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Papadopoulou, N.; Kempuraj, D.; Boucher, W.S.; Sugimoto, K.; Cetrulo, C.L.; Theoharides, T.C. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J. Immunol. 2005, 174, 7665–7675. [Google Scholar] [CrossRef] [Green Version]
- Saban, R. Angiogenic factors, bladder neuroplasticity and interstitial cystitis-new pathobiological insights. Transl. Androl. Urol. 2015, 4, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Boucher, W.; Kempuraj, D.; Michaelian, M.; Theoharides, T.C. Corticotropin-releasing hormone-receptor 2 is required for acute stress-induced bladder vascular permeability and release of vascular endothelial growth factor. BJU Int. 2010, 106, 1394–1399. [Google Scholar] [CrossRef]
- Hauser, P.J.; VanGordon, S.B.; Seavey, J.; Sofinowski, T.M.; Ramadan, M.; Abdullah, S.; Buffington, C.A.; Hurst, R.E. Abnormalities in Expression of Structural, Barrier, and Differentiation Related Proteins and Chondroitin Sulfate in the Urothelium of Cats with Feline Interstitial Cystitis Mimic Those Seen in Human Interstitial Cystitis. J. Urol. 2015, 194, 571–577. [Google Scholar] [CrossRef]
- Birder, L.; Andersson, K.E. Urothelial signaling. Physiol. Rev. 2013, 93, 653–680. [Google Scholar] [CrossRef] [Green Version]
- Sanchez Freire, V.; Burkhard, F.C.; Kessler, T.M.; Kuhn, A.; Draeger, A.; Monastyrskaya, K. MicroRNAs may mediate the down-regulation of neurokinin-1 receptor in chronic bladder pain syndrome. Am. J. Pathol. 2010, 176, 288–303. [Google Scholar] [CrossRef]
- Yu, A.S.; Cheng, M.H.; Angelow, S.; Günzel, D.; Kanzawa, S.A.; Schneeberger, E.E.; Fromm, M.; Coalson, R.D. Molecular basis for cation selectivity in claudin-2-based paracellular pores: Identification of an electrostatic interaction site. J. Gen. Physiol. 2009, 133, 111–127. [Google Scholar] [CrossRef] [Green Version]
- Rickard, A.; Dorokhov, N.; Ryerse, J.; Klumpp, D.J.; McHowat, J. Characterization of tight junction proteins in cultured human urothelial cells. Vitr. Cell Dev. Biol. Anim. 2008, 44, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Zhang, J.; Zhou, F.; Zhang, P. Increased Transient Receptor Potential Melastatin 8 Expression in the Development of Bladder Pain in Patients With Interstitial Cystitis/Bladder Pain Syndrome. Urology 2020, 146, 301.e1–301.e6. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, J.; Schulte-Baukloh, H.; Stolzenburg, J.U.; Speroni di Fenizio, P.; Horn, L.C.; Ruffert, H.; Hartenstein, S.; Burger, M.; Schulze, M.; Schwalenberg, T. Individual receptor profiling as a novel tool to support diagnosis of bladder pain syndrome/interstitial cystitis (BPS/IC). World J. Urol. 2012, 30, 693–700. [Google Scholar] [CrossRef] [PubMed]
| Comorbidity | References |
|---|---|
| Allergies and autoimmune disorders | [9] |
| Asthma (especially the non-allergic type) | [10] |
| Sjögrens’s syndrome | [11,12,13] |
| Atopic dermatitis | [9] |
| Lupus erythematosus | [14] |
| Fibromyalgia | [9,13,15] |
| Rheumatoid arthritis | [9,15] |
| Chronic fatigue | [13] |
| Endometriosis | [16] |
| Irritable bowel syndrome, Colitis ulcerosa | [9,17] |
| Hashimoto’s thyroiditis and Hyperthyroidism | [9,15,18] |
| Psoriasis | [9] |
| Focus | Marker | Gene/Protein | Effect | References |
|---|---|---|---|---|
| Predisposition | Chromosome | [22] | ||
| 3p13-p12.3 | CNTN3 | sensory processing/pain | ||
| 1p21-q25 | NGF | nerve proliferation | ||
| IL6 | inflammation | |||
| CRP | inflammation | |||
| 3p21.1-p14.3 | *CACNA2D3 | neural activity (brain) | ||
| 4q12-q13 | *PDGFRA | proliferation, development | ||
| 9p24-p22 | IL33 | innate immunity, mast cell activation/proliferation | ||
| 14q24-q31 | FOS | inflammation | ||
| SNP | ||||
| rs11127292 | MYT1L | neuronal differentiation | [23] | |
| rs6311 | HTR2A | pain | ||
| rs1799971 | OPRM1 | pain | ||
| Early detection | PAF and PAFR | iNOS, COX-1, IL-6, TNF | urothelial barrier and vascular integrity | [54] |
| CRH-VEGF-axis CRHR | microvessel formation | [61] | ||
| Differential | CLCN3 | CLCN3 | pain | [24] |
| diagnosis | S-100 gene family | S-100 proteins | inflammation | |
| (HIC vs. NHIC) | E2F1 | E2F1 | cell cycle | |
| lncRNA MEG3 | TLR7 | immunity | [32] | |
| miR-19a-3p | MEG3 | immunity | ||
| B-lymphocytes, plasma cells | clonal expansion infiltration | [6] | ||
| CRHR | vascular integrity, microvessel formation | [59] | ||
| Diagnosis/Prognosis | miR-320 family | E2F1/2, TUBTACR1/2 | cell cycle pain | [26] |
| miR-199a-5p | PALS1 | tight junction formation, urothelial cell polarity | [27,28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neuhaus, J.; Berndt-Paetz, M.; Gonsior, A. Biomarkers in the Light of the Etiopathology of IC/BPS. Diagnostics 2021, 11, 2231. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11122231
Neuhaus J, Berndt-Paetz M, Gonsior A. Biomarkers in the Light of the Etiopathology of IC/BPS. Diagnostics. 2021; 11(12):2231. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11122231
Chicago/Turabian StyleNeuhaus, Jochen, Mandy Berndt-Paetz, and Andreas Gonsior. 2021. "Biomarkers in the Light of the Etiopathology of IC/BPS" Diagnostics 11, no. 12: 2231. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics11122231