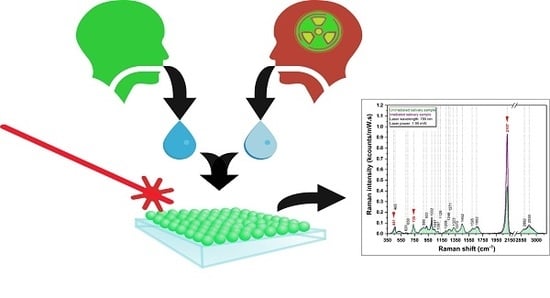
The Effects of Low-Dose Irradiation on Human Saliva: A Surface-Enhanced Raman Spectroscopy Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Saliva Sampling
2.2. Synthesis of Silver Nanoparticles (NPs)
2.3. SERS Substrate Preparation
2.4. UV–VIS Absorption Measurements
2.5. Transmission Electron Microscope (TEM) Measurements
2.6. Atomic Force Microscopy (AFM) Measurements
2.7. Raman/SERS Measurements and Data Collection
3. Results and Discussion
3.1. Synthesis and Characterization of Solid SERS Substrates
3.2. Raman Spectrum of Saliva
3.3. SERS Spectrum of Saliva
3.3.1. SERS Spectrum of Healthy Donors
3.3.2. SERS Spectrum of Irradiated Salivary Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pernot, E.; Hall, J.; Baatout, S.; Benotmane, M.A.; Blanchardon, E.; Bouffler, S.; El Saghire, H.; Gomolka, M.; Guertler, A.; Harms-Ringdahl, M.; et al. Ionizing radiation biomarkers for potential use in epidemiological studies. Mutat. Res. 2012, 751, 258–286. [Google Scholar] [CrossRef] [PubMed]
- Jennings, F.L. Mammalian Radiation Lethality: A Disturbance in Cellular Kinetics. Jama J. Am. Med. Assoc. 1966, 196, 743. [Google Scholar] [CrossRef]
- Eric, J.; Hall, A.J.G. Radiobiology for the Radiologist; Lippincottt Williams & Wilkins: Philadelphia, PA, USA, 2000; ISBN 9781469821634. [Google Scholar]
- Fazel, R.; Krumholz, H.M.; Wang, Y.; Ross, J.S.; Chen, J.; Ting, H.H.; Shah, N.D.; Nasir, K.; Einstein, A.J.; Nallamothu, B.K. Exposure to Low-Dose Ionizing Radiation from Medical Imaging Procedures. N. Engl. J. Med. 2009, 361, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Oenning, A.C.; Jacobs, R.; Pauwels, R.; Stratis, A.; Hedesiu, M.; Salmon, B.; DIMITRA Research Group. Cone-beam CT in paediatric dentistry: DIMITRA project position statement. Pediatr. Radiol. 2018, 48, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Kapila, S.D.; Nervina, J.M. CBCT in orthodontics: Assessment of treatment outcomes and indications for its use. Dentomaxillofacial Radiol. 2015, 44, 20140282. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, P.; Hollender, L.; Bollen, A.-M.; Young, J.D.; McGee, M.; Grosso, A. Head-and-neck organ doses from an episode of orthodontic care. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, M.; Auvinen, A.; Cardis, E.; Durante, M.; Harms-Ringdahl, M.; Jourdain, J.R.; Madas, B.G.; Ottolenghi, A.; Pazzaglia, S.; Prise, K.M.; et al. Multidisciplinary European Low Dose Initiative (MELODI): Strategic research agenda for low dose radiation risk research. Radiat. Environ. Biophys. 2018, 57, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Pernot, E.; Cardis, E.; Badie, C. Usefulness of Saliva Samples for Biomarker Studies in Radiation Research. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Duan, Y. Saliva: A potential media for disease diagnostics and monitoring. Oral Oncol. 2012, 48, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Theodorakou, C.; Walker, A.; Horner, K.; Pauwels, R.; Bogaerts, R.; Jacobs Dds, R.; SEDENTEXCT Project Consortium. Estimation of paediatric organ and effective doses from dental cone beam CT using anthropomorphic phantoms. Br. J. Radiol. 2012, 85, 153–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonne, N.J.; Wong, D.T. Salivary biomarker development using genomic, proteomic and metabolomic approaches. Genome Med. 2012, 4, 82. [Google Scholar] [CrossRef]
- Vandenabeele, P. Practical Raman Spectroscopy: An Introduction; John Wiley & Sons: Chichester, UK, 2013; ISBN 9781119961901. [Google Scholar]
- Grasselli, J. Chemical Applications of Raman Spectroscopy; John Wiley & Sons: New York, NY, USA, 1981. [Google Scholar]
- Simon, I.; Hedesiu, M.; Virag, P.; Salmon, B.; Tarmure, V.; Baciut, M.; Bran, S.; Jacobs, R.; Falamas, A. Raman Micro-Spectroscopy of Dental Pulp Stem Cells: An Approach to Monitor the Effects of Cone Beam Computed Tomography Low-Dose Ionizing Radiation. Anal. Lett. 2019, 52, 1097–1111. [Google Scholar] [CrossRef]
- Bonifacio, A.; Cervo, S.; Sergo, V. Label-free surface-enhanced Raman spectroscopy of biofluids: Fundamental aspects and diagnostic applications. Anal. Bioanal. Chem. 2015, 407, 8265–8277. [Google Scholar] [CrossRef] [PubMed]
- Schlücker, S. Surface Enhanced Raman Spectroscopy: Analytical, Biophysical and Life Science Applications; Wiley-VCH: Weinheim, Germany, 2011; ISBN 3527633065. [Google Scholar]
- Leopold, N.; Bernhard, L. A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Silver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Connolly, J.M.; Davies, K.; Kazakeviciute, A.; Wheatley, A.M.; Dockery, P.; Keogh, I.; Olivo, M. Non-invasive and label-free detection of oral squamous cell carcinoma using saliva surface-enhanced Raman spectroscopy and multivariate analysis. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Huang, S.; Lin, D.; Chen, G.; Xu, Y.; Li, Y.; Huang, Z.; Pan, J.; Chen, R.; Zeng, H. Surface-enhanced Raman spectroscopy of saliva proteins for the noninvasive differentiation of benign and malignant breast tumors. Int. J. Nanomed. 2015, 10, 537–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, S.; Xu, Y.; Huang, L.; Zheng, W.; Huang, C.; Huang, S.; Lin, J.; Lin, D.; Feng, S.; Chen, R.; et al. Non-invasive detection of nasopharyngeal carcinoma using saliva surface-enhanced Raman spectroscopy. Oncol. Lett. 2016, 11, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, T.; Lin, J. Spectral analysis of human saliva for detection of lung cancer using surface-enhanced Raman spectroscopy. J. Biomed. Opt. 2012, 17, 037003. [Google Scholar] [CrossRef]
- Stefancu, A.; Badarinza, M.; Moisoiu, V.; Iancu, S.D.; Serban, O.; Leopold, N.; Fodor, D. SERS-based liquid biopsy of saliva and serum from patients with Sjögren’s syndrome. Anal. Bioanal. Chem. 2019, 1–7. [Google Scholar] [CrossRef]
- Chandler, J.D.; Day, B.J. THIOCYANATE: A potentially useful therapeutic agent with host defense and antioxidant properties. Biochem. Pharm. 2012, 84, 1381–1387. [Google Scholar] [CrossRef] [Green Version]
- Olea, F.; Parras, P. Determination of Serum Levels of Dietary Thiocyanate. J. Anal. Toxicol. 1992, 16, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.M.; Hamza, M.S.A.; Kelany, A.E. A novel spectrophotometric method for batch and flow injection determination of cyanide in electroplating wastewater. Talanta 2007, 71, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Michigami, Y.; Fujii, K.; Ueda, K.; Yamamoto, Y. Determination of thiocyanate in human saliva and urine by ion chromatography. Analyst 1992, 117, 1855. [Google Scholar] [CrossRef] [PubMed]
- Blount, B.C.; Özpinar, A.; Alwis, K.U.; Caudill, S.P.; Gillespie, J.R. Perchlorate, Nitrate, Thiocyanate, and Iodide Levels in Chicken Feed, Water, and Eggs from Three Farms. J. Agric. Food Chem. 2008, 56, 10709–10715. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yu, H.; Wang, Y. Rapid and Simultaneous Determination of Tetrafluoroborate, Thiocyanate and Hexafluorophosphate by High-Performance Liquid Chromatography Using a Monolithic Column and Direct Conductivity Detection. Anal. Sci. 2010, 26, 861–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, T.; Liu, Y.; Xu, L.; Wu, Y.; Ying, Y.; Wen, Y.; Guo, X.; Yang, H. Au dotted magnetic graphene sheets for sensitive detection of thiocyanate. Sens. Actuators B Chem. 2017, 241, 376–382. [Google Scholar] [CrossRef]
- Tsuge, K.; Kataoka, M.; Seto, Y. Cyanide and Thiocyanate Levels in Blood and Saliva of Healthy Adult Volunteers. J. Health Sci. 2000, 46, 343–350. [Google Scholar] [CrossRef]
- Aggarwal, A.; Keluskar, V.; Goyal, R.; Dahiya, P. Salivary thiocyanate: A biochemical indicator of cigarette smoking in adolescents. Oral Health Prev. Dent. 2013, 11, 221–227. [Google Scholar]
- Marcu, M.; Hedesiu, M.; Salmon, B.; Pauwels, R.; Stratis, A.; Oenning, A.C.C.; Cohen, M.E.; Jacobs, R.; Baciut, M.; Roman, R.; et al. Estimation of the radiation dose for pediatric CBCT indications: A prospective study on ProMax3D. Int. J. Paediatr. Dent. 2018, 28, 300–309. [Google Scholar] [CrossRef]
- Munro, C.L.; Grap, M.J.; Jablonski, R.; Boyle, A. Oral health measurement in nursing research: State of the science. Biol. Res. Nurs. 2006, 8, 35–42. [Google Scholar] [CrossRef]
- Dorney, K.M.; Baker, J.D.; Edwards, M.L.; Kanel, S.R.; O’Malley, M.; Pavel Sizemore, I.E. Tangential Flow Filtration of Colloidal Silver Nanoparticles: A “Green” Laboratory Experiment for Chemistry and Engineering Students. J. Chem. Educ. 2014, 91, 1044–1049. [Google Scholar] [CrossRef]
- Thanu, D.P.R.; Srinadhu, E.S.; Zhao, M.; Dole, N.V.; Keswani, M. Fundamentals and Applications of Plasma Cleaning. In Developments in Surface Contamination and Cleaning: Applications of Cleaning Techniques; Elsevier: Amsterdam, The Netherlands, 2019; pp. 289–353. [Google Scholar]
- Bonifacio, A.; Dalla Marta, S.; Spizzo, R.; Cervo, S.; Steffan, A.; Colombatti, A.; Sergo, V. Surface-enhanced Raman spectroscopy of blood plasma and serum using Ag and Au nanoparticles: A systematic study. Anal. Bioanal. Chem. 2014, 406, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, J.S.; Malissek, M.; Simon, S.; Knauer, S.K.; Maskos, M.; Stauber, R.H.; Peukert, W.; Treuel, L. Impact of the Nanoparticle–Protein Corona on Colloidal Stability and Protein Structure. Langmuir 2012, 28, 9673–9679. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.D.; Chan, W.C.W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef] [PubMed]
- Virkler, K.; Lednev, I.K. Forensic body fluid identification: The Raman spectroscopic signature of saliva. Analyst 2010, 135, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Gonchukov, S.; Sukhinina, A.; Bakhmutov, D.; Minaeva, S. Raman spectroscopy of saliva as a perspective method for periodontitis diagnostics. Laser Phys. Lett. 2012, 9, 73–77. [Google Scholar] [CrossRef]
- Xiao, G.-N.; Man, S.-Q. Surface-enhanced Raman scattering of methylene blue adsorbed on cap-shaped silver nanoparticles. Chem. Phys. Lett. 2007, 447, 305–309. [Google Scholar] [CrossRef]
- Anyu, C.; Lin, H.; Jinghua, L.; ZiJian, C.; Yi, J.; Dian, Q.; Xun, G.; Chunwei, L.; Wen, H.; Hong, W. Detecting Narcotic Usage Using Surface-Enhanced Raman Spectroscopy on Saliva Samples. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Munich, Germany, 7–12 September 2009; Springer: Berlin/Heidelberg, Germany, 2009; pp. 71–74. [Google Scholar]
- Kah, J.C.Y.; Kho, K.W.; Lee, C.G.L.; James, C.; Sheppard, R.; Shen, Z.X.; Soo, K.C.; Olivo, M.C. Early diagnosis of oral cancer based on the surface plasmon resonance of gold nanoparticles. Int. J. Nanomed. 2007, 2, 785–798. [Google Scholar]
- Farquharson, S.; Shende, C.; Inscore, F.E.; Maksymiuk, P.; Gift, A. Analysis of 5-fluorouracil in saliva using surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2005, 36, 208–212. [Google Scholar] [CrossRef]
- de Almeida, P.D.V.; Grégio, A.M.T.; Machado, M.A.N.; de Lima, A.A.S.; Azevedo, L.R. Saliva composition and functions: A comprehensive review. J. Contemp. Dent. Pract. 2008, 9, 72–80. [Google Scholar]
- Ashby, M.T. Inorganic Chemistry of Defensive Peroxidases in the Human Oral Cavity. J. Dent. Res. 2008, 87, 900–914. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Nicholls, S.J.; Rodriguez, E.R.; Kummu, O.; Hörkkö, S.; Barnard, J.; Reynolds, W.F.; Topol, E.J.; DiDonato, J.A.; Hazen, S.L. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 2007, 13, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.E.; Pattison, D.I.; Talib, J.; Summers, F.A.; Harmer, J.A.; Celermajer, D.S.; Hawkins, C.L.; Davies, M.J. High plasma thiocyanate levels in smokers are a key determinant of thiol oxidation induced by myeloperoxidase. Free Radic. Biol. Med. 2011, 51, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Lord, R.C.; Mendelsohn, R. Laser-excited Raman spectroscopy of biomolecules. V. Conformational changes associated with the chemical denaturation of lysozyme. J. Am. Chem. Soc. 1974, 96, 3038–3042. [Google Scholar] [CrossRef] [PubMed]
- Remmele, R.L.; McMillan, P.; Bieber, A. Raman spectroscopic studies of hen egg-white lysozyme at high temperatures and pressures. J. Protein Chem. 1990, 9, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.A.; Junge, J.M.; Fisher, E.H. The amino acid composition of alpha-amylase from Aspergillus oryzae. J. Biol. Chem. 1960, 235, 371–378. [Google Scholar]
- Bianchetta, J.D.; Bidaud, J.; Guidoni, A.A.; Bonicel, J.J.; Rovery, M. Porcine pancreatic lipase. Sequence of the first 234 amino acids of the peptide chain. Eur. J. Biochem. 1979, 97, 395–405. [Google Scholar] [CrossRef]
- Vanni, S. Raman Chemical Imaging Provides Rapid, Non-Invasive and Reagentless Biothreat Detection; ChemImage Corporation: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Kalasinsky, K.S.; Hadfield, T.; Shea, A.A.; Kalasinsky, V.F.; Nelson, M.P.; Neiss, J.; Drauch, A.J.; Vanni, G.S.; Treado, P.J. Raman Chemical Imaging Spectroscopy Reagentless Detection and Identification of Pathogens: Signature Development and Evaluation. Anal. Chem. 2007, 79, 2658–2673. [Google Scholar] [CrossRef]
- Stiufiuc, R.; Iacovita, C.; Stiufiuc, G.; Florea, A.; Achim, M.; Lucaciu, C.M. A new class of pegylated plasmonic liposomes: Synthesis and characterization. J. Colloid Interface Sci. 2015, 437, 17–23. [Google Scholar] [CrossRef]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Barrett, T.W. Laser Raman spectra of mono-, oligo- and polysaccharides in solution. Spectrochim. Acta Part. Mol. Spectrosc. 1981, 37, 233–239. [Google Scholar] [CrossRef]
- Khaustova, S.; Shkurnikov, M.; Tonevitsky, E.; Artyushenko, V.; Tonevitsky, A. Noninvasive biochemical monitoring of physiological stress by Fourier transform infrared saliva spectroscopy. Analyst 2010, 135, 3183. [Google Scholar] [CrossRef]
- Bron, M.; Holze, R. The adsorption of thiocyanate ions at gold electrodes from an alkaline electrolyte solution: A combined in situ infrared and raman spectroscopic study. Electrochimica Acta 1999, 45, 1121–1126. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Z.; Zong, S.; Cui, Y. Rapid and reproducible analysis of thiocyanate in real human serum and saliva using a droplet SERS-microfluidic chip. Biosens. Bioelectron. 2014, 62, 13–18. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, F.; Wang, D.; Ma, P.; Gao, D.; Han, J.; Li, Y.; Yu, A.; Song, D.; Wang, X. Simultaneous determination of thiocyanate ion and melamine in milk and milk powder using surface-enhanced Raman spectroscopy. Anal. Methods 2014, 6, 8388–8395. [Google Scholar] [CrossRef]
- Wong, M. Surface-Enhanced Raman Spectroscopy for Forensic Analysis of Human Saliva. 2017. Available online: https://open.bu.edu/handle/2144/23997 (accessed on 20 August 2019).







| Raman Band (cm−1) | Vibrational Mode | Assignment |
|---|---|---|
| 441 | S−C≡N bending vibration | Thiocyanate |
| 620 | C-C twisting mode | Phenylalanine |
| 650 | C-C twisting mode | Phenylalanine |
| 735 | C−S stretching vibration | Thiocyanate |
| 880 | v (C-C) | Hydroxyproline |
| 1002 | νS (C-C) | Phenylalanine |
| 1047 | ν (C-O), ν (C-N) | Proteins |
| 1208 | ν (C-C6H5) | Tryptophan |
| 1452 | δ (C-H) | Collagen, lipids |
| 1661 | ν (C=C) | Amide I |
| 2107 | −C≡N stretching vibration | Thiocyanate |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colceriu-Șimon, I.M.; Hedeșiu, M.; Toma, V.; Armencea, G.; Moldovan, A.; Știufiuc, G.; Culic, B.; Țărmure, V.; Dinu, C.; Berindan-Neagoe, I.; et al. The Effects of Low-Dose Irradiation on Human Saliva: A Surface-Enhanced Raman Spectroscopy Study. Diagnostics 2019, 9, 101. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics9030101
Colceriu-Șimon IM, Hedeșiu M, Toma V, Armencea G, Moldovan A, Știufiuc G, Culic B, Țărmure V, Dinu C, Berindan-Neagoe I, et al. The Effects of Low-Dose Irradiation on Human Saliva: A Surface-Enhanced Raman Spectroscopy Study. Diagnostics. 2019; 9(3):101. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics9030101
Chicago/Turabian StyleColceriu-Șimon, Ioana Maria, Mihaela Hedeșiu, Valentin Toma, Gabriel Armencea, Alin Moldovan, Gabriela Știufiuc, Bogdan Culic, Viorica Țărmure, Cristian Dinu, Ioana Berindan-Neagoe, and et al. 2019. "The Effects of Low-Dose Irradiation on Human Saliva: A Surface-Enhanced Raman Spectroscopy Study" Diagnostics 9, no. 3: 101. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics9030101