Investigating Permeability of Coal Samples of Various Porosities under Stress Conditions
Abstract
:1. Introduction
2. Methodology
2.1. Technical Analyses of Coal
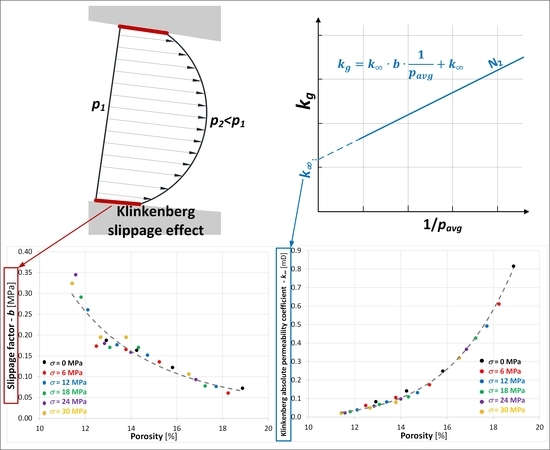
2.2. Describing the Phenomenon of Coal Permeability in Relation to Gas
2.3. Measurement Stand for Studies into Permeability of Coal under Stress Conditions
2.4. Measurement Procedure
- (1)
- Outgassing the sample to a vacuum 10−5 bar for 24 h;
- (2)
- Measurement of the sample’s permeability of a particular gas (N2 or CH4), encompassing two subs-stages:
- 2.1
- Applying stress on the sample (0, 6, 12, 18, 24, 30 MPa);
- 2.2
- Injecting the gas under specific measurement conditions (pressure at the sample’s inlet and outlet) and waiting for the flow to stabilize, with the process repeated 5–8 times under various measurement conditions (by changing the pressure at the sample’s inlet and outlet).
- the gas pressure values at the inlet and outlet of the sample measured by the and pressure transducers,
- the gas flow rate measured by the gas flow meter,
- the atmospheric pressure measured by the barometer .
3. Research Material
4. Results
- Darcy’s permeability coefficients of coal briquettes, as depending on the average pressure of nitrogen and methane,
- the Klinkenberg absolute permeability coefficients of coal briquettes and the Klinkenberg slippage factors , as depending on stress and coal porosity,
- the Klinkenberg permeability parameters ratio to nitrogen and methane.
4.1. Darcy’s Permeability Coefficients
4.2. The Klinkenberg Permeability Parameters and
4.3. The Klinkenberg Permeability Parameters Ratio to Nitrogen and Methane
5. Conclusions
- the values of the Darcy permeability coefficients decrease as the average gas pressure in a coal sample increases,
- the values of the Klinkenberg permeability coefficient decrease along with an increase in stress and the corresponding reduction in porosity,
- reduction of the porosity of the samples from 18.87% to 11.42% caused: (i) a 40-time decrease (from 0.816 mD to 0.021 mD) in the value of the Klinkenberg permeability coefficient in relation to nitrogen, and (ii) a 5-time increase (from 0.072 MPa to 0.324 MPa) in the value of the slippage factor ,
- reduction in the porosity of the samples from 14.14% to 7.41% caused: (i) a ca. 20-time decrease (from 0.01031 mD to 0.00052 mD) in the value of the Klinkenberg permeability coefficient in relation to methane, and (ii) a 1.5-time increase (from 0.171 MPa to 0.267 MPa) in the value of the slippage factor ,
- the permeability of the investigated coal to nitrogen exceeded its permeability to methane by up to one order of magnitude (6.6 times for the porosity of ca. 11.3%, and 14 times for the porosity of ca. 13.7%),
- the slippage factor parameter for the investigated coal reached similar values using nitrogen and methane, within the investigated range of the coal briquettes’ porosities,
- along with an increase in the porosity of briquettes, the Klinkenberg slippage effect: (i) disappeared in the case of nitrogen, (ii) and recorded a slight decline in value in the case of methane,
- the stress applied to coal samples resulted predominantly in a change of their porosity; that porosity was the main factor influencing the nature of the permeability process described by the Klinkenberg Equation (6).
- The conducted tests into the permeability of coal briquettes of various porosities demonstrate that the stress applied to samples resulted predominantly in a change of the porosity of these samples. The present research shows, in an experimental manner, that the porosity is the parameter with a deciding impact on permeability as described by Klinkenberg.
Funding
Conflicts of Interest
References
- Kreiner, K.; Żyła, M. Binary character of surface of coal. Gór. Geoinż. 2006, 30, 19–34. [Google Scholar]
- Clarkson, C.R.; Bustin, R.M. The effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modelling study: 1. Isotherms and pores volume distributions. Fuel 1999, 78, 1333–1344. [Google Scholar] [CrossRef]
- Mastalerz, M.; Gluskoter, H.; Rupp, J. Carbon dioxide and methane sorption in high volatile bituminous coals from Indians, USA. Int. J. Coal Geol. 2004, 60, 43–55. [Google Scholar] [CrossRef]
- Mahajan, O.P.; Walker, J.P.L. Porosity of Coal and Coals Products; Karr, C., Jr., Ed.; Academic Press: New York, NY, USA, 1978; Volume 1, p. 125. [Google Scholar]
- Ettinger, J.L. Solubility of Methane Contained in Coal Deposits. Arch. Min. Sci. 1990, 33, 35. [Google Scholar]
- Battistutta, E.; van Hemert, P.; Lutynski, M.; Bruining, H.; Wolf, K.H. Swelling and sorption experiments on methane, nitrogen and carbon dioxide on dry Selar Cornish coal. Int. J. Coal Geol. 2010, 84, 39–48. [Google Scholar] [CrossRef]
- Żyła, M. Układ węgiel kamienny-metan w aspekcie desorpcji i odzyskiwania metanu z gazów kopalnianych; Uczelniane Wydawnictwo Naukowo-Badawcze: AGH, Kraków, 2000. [Google Scholar]
- Gawor, M.; Skoczylas, N. Sorption rate of carbon dioxide on coal. Transp. Porous Media 2014, 101, 269–279. [Google Scholar] [CrossRef]
- Pajdak, A. Parameters of N2 and CO2 adsorption onto coal at various temperatures. In Proceedings of the 18th International Multidisciplinary Scientific Geoconference SGEM, Albena, Bulgaria, 30 June–9 July 2018; pp. 633–640. [Google Scholar]
- Chattaraj, S.; Mohanty, D.; Kumar, T.; Halder, G. Thermodynamics, kinetics and modeling of sorption behaviour of coalbed methane—A review. J. Unconv. Oil Gas Resour. 2016, 16, 14–33. [Google Scholar] [CrossRef]
- Kudasik, M. Results of comparative sorption studies of the coal-methane system carried out by means of an original volumetric device and a reference gravimetric instrument. Adsorpt. J. Int. Adsorpt. Soc. 2017, 23, 613–626. [Google Scholar] [CrossRef]
- Larsen, J.W. The effects of dissolved CO2 on coal structure and properties. Int. J. Coal Geol. 2004, 57, 63–70. [Google Scholar] [CrossRef]
- Karacan, C.O. Swelling-induced strains internal to a stressed coal associated with CO2 sorption. Int. J. Coal Geol. 2007, 72, 209–220. [Google Scholar] [CrossRef]
- Hol, S.; Peach, C.J.; Spiers, C.J. Applied stress reduces the CO2 sorption capacity of coal. Int. J. Coal Geol. 2011, 85, 128–142. [Google Scholar] [CrossRef]
- Day, S.; Fry, R.; Sakurovs, R. Swelling of coal in carbon dioxide, methane and their mixtures. Int. J. Coal Geol. 2012, 93, 40–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Lebedev, M.; Sarmadivaleh, M.; Barifcani, A.; Rahman, T.; Iglauer, S. Swelling effect on coal micro structure and associated permeability reduction. Fuel 2016, 182, 568–576. [Google Scholar] [CrossRef]
- Han, F.; Chen, G.; Liu, Z.; Yang, J. Correlation of swelling and sorption properties of block coal sample. Fuel 2017, 188, 452–461. [Google Scholar] [CrossRef]
- Zang, J.; Wang, K. Gas sorption-induced coal swelling kinetics and its effects on coal permeability evolution: Model development and analysis. Fuel 2017, 189, 164–177. [Google Scholar] [CrossRef]
- Walker, J.P.L.; Verma, S.K.; Rivera-Utrilla, J.; Davis, A. Densities, porosities and surface areas of coal macerals as measured by their interaction with gases, vapours and liquids. Fuel 1988, 67, 1615–1623. [Google Scholar] [CrossRef]
- Reucroft, P.J.; Patel, H. Gas-induced swelling in coal. Fuel 1986, 65, 816–820. [Google Scholar] [CrossRef]
- Siriwardane, H.J.; Gondle, R.K.; Smith, D.H. Shrinkage and swelling of coal induced by desorption and sorption of fluids: Theoretical model and interpretation of field project. Int. J. Coal Geol. 2009, 77, 90–102. [Google Scholar] [CrossRef]
- Czerw, K. Methane and carbon dioxide sorption/desorption on bituminous coal—Experiments on cubicoid sample cut from the primal coal lump. Int. J. Coal Geol. 2011, 85, 72–77. [Google Scholar] [CrossRef]
- Li, Y.; Tang, D.; Xu, H.; Meng, Y.; Li, J. Experimental research on coal permeability: The roles of effective stress and gas slippage. J. Nat. Gas Sci. Eng. 2014, 21, 481–488. [Google Scholar] [CrossRef]
- Majewska, Z.; Majewski, S.; Ziętek, J. Swelling and acoustic emission behaviour of unconfined and confined coal during sorption of CO2. Int. J. Coal Geol. 2013, 116–117, 17–25. [Google Scholar] [CrossRef]
- Xu, B.X.; Li, X.F.; Haghighi, M.; Ren, W.N.; Du, X.Y.; Chen, D.; Zhai, Y.Y. Optimization of hydraulically fractured well configuration in anisotropic coal-bed methane reservoirs. Fuel 2013, 107, 859–865. [Google Scholar] [CrossRef]
- Aguilera, R.F.; Ripple, R.D.; Aguilera, R. Link between endowments, economics and environment in conventional and unconventional gas reservoirs. Fuel 2014, 126, 224–238. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, J.; Hou, Q.; Niu, Q.; Tian, J.; Wang, H.; Fu, X. Changes in the anisotropic permeability of low-rank coal under varying effective stress in Fukang mining area, China. Fuel 2018, 234, 1481–1497. [Google Scholar] [CrossRef]
- Chen, Z.; Pan, Z.; Liu, J.; Connell, L.D.; Elsworth, D. Effect of the effective stress coefficient and sorption-induced strain on the evolution of coal permeability: Experimental observations. Int. J. Greenh. Gas Control 2011, 5, 1284–1293. [Google Scholar] [CrossRef]
- Konecny, P.; Kozusnikova, A. Influence of stress on the permeability of coal and sedimentary rocks of the Upper Silesian basin. Int. J. Rock Mech. Min. Sci. 2011, 48, 347–352. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Choi, S.K. Coal cleat permeability for gas movement under triaxial, non-zero lateral strain condition: Atheoretical and experimental study. Fuel 2013, 109, 389–399. [Google Scholar] [CrossRef]
- Meng, Z.; Li, G. Experimental research on the permeability of high-rank coal under a varying stress and its influencing factors. Eng. Geol. 2013, 162, 108–117. [Google Scholar] [CrossRef]
- Zou, J.; Chen, W.; Yang, D.; Yu, H.; Yuan, J. The impact of effective stress and gas slippage on coal permeability under cyclic loading. J. Nat. Gas Sci. Eng. 2016, 31, 236–248. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, K.; Zhou, H.; Cheng, Y.; Zhang, H.; Wang, L. Experimental investigation into the damage-induced permeability and deformation relationship of tectonically deformed coal from Huainan coalfield, China. J. Nat. Gas Sci. Eng. 2018, 60, 202–213. [Google Scholar] [CrossRef]
- Huy, P.Q.; Sasaki, K.; Sugai, Y.; Ichikawa, S. Carbon dioxide gas permeability of coal core samples and estimation of fracture aperture width. Int. J. Coal Geol. 2010, 83, 1–10. [Google Scholar] [CrossRef]
- Somerton, W.H.; Söylemezoglu, I.M.; Dudley, R.C. Effect of stress on permeability of coal. Int. J. Rock Mech. Min. Sci. Geomech. Abstr. 1975, 12, 129–145. [Google Scholar] [CrossRef]
- Durucan, S.; Edwards, J.S. The effects of stress and fracturing on permeability of coal. Min. Sci. Technol. 1986, 3, 205–216. [Google Scholar] [CrossRef]
- Pajdak, A.; Godyń, K.; Kudasik, M.; Murzyn, T. The use of selected research methods to describe the pore space of dolomite from copper ore mine, Poland. Environ. Earth Sci. 2017, 76, 389. [Google Scholar] [CrossRef]
- Dake, L.P. Fundamentals of Reservoir Engineering. No. 8. Amsterdam: Developments in Petroleum Science; Elsevier Science BV: New York, NY, USA, 1978. [Google Scholar]
- Klinkenberg, L.J. The permeability of porous media to liquids and gases. In Proceedings of the API Drilling and Production Practice, New York, NY, USA, 1 January 1941; pp. 200–213. [Google Scholar]
- Scheidegger, A.E. The Physics of Flow through Porous Media, 3rd ed.; University of Toronto Press: Toronto, ON, Canada, 1974. [Google Scholar]
- Skoczylas, N.; Kudasik, M.; Wierzbicki, M.; Murzyn, T. New instruments and methods for analysing the coal-methane system. Stud. Geotech. Mech. 2015, 37, 85–91. [Google Scholar] [CrossRef]
- Skoczylas, N. Analyzing the parameters of the coal–gas system using a low-cost device based on a flowmeter. Adsorpt. Sci. Technol. 2015, 33, 769–782. [Google Scholar] [CrossRef]
- Skoczylas, N. Determining the gas permeability coefficient of a porous medium by means of the bubble-counting flow meter. Meas. Sci. Technol. 2015, 26, 085004. [Google Scholar] [CrossRef]
- Kudasik, M. The manometric sorptomat—An innovative volumetric instrument for sorption measurements performed under isobaric conditions. Meas. Sci. Technol. 2016, 27, 035903. [Google Scholar] [CrossRef]
- Kudasik, M.; Skoczylas, N. Analyzer for measuring gas contained in the pore space of rocks. Meas. Sci. Technol. 2017, 28, 105901. [Google Scholar] [CrossRef] [Green Version]
- Skoczylas, N.; Wierzbicki, M.; Kudasik, M. A simple method for measuring basic parameters of the coal-methane system under mining conditions. J. Min. Sci. 2018, 3, 186–197. [Google Scholar]
- Kudasik, M.; Pajdak, A.; Skoczylas, N. The validation process of the method of balancing gas contained in the pore space of rocks via rock comminution. Arch. Min. Sci. 2018, 63, 989–1005. [Google Scholar]
- Kanciruk, A. SGM-1C measuring instruments: their modifications and examples of application. Elektron. Podzesp. Zastos. Electron. 1998, 12, 20–22. [Google Scholar]
- Kudasik, M.; Skoczylas, N.; Sobczyk, J.; Topolnicki, J. Manostat—An accurate gas pressure regulator. Meas. Sci. Technol. 2010, 21, 085402. [Google Scholar] [CrossRef]
- Topolnicki, J.; Kudasik, M.; Skoczylas, N.; Sobczyk, J. Low cost capillary flow meter. Sens. Actuators A Phys. 2009, 152, 146–150. [Google Scholar] [CrossRef]
- Komorek, J.; Lewandowska, M.; Probierz, K. Peculiarities of petrographic composition of coking coals in southwest part of Upper Silesian Coal Basin (Poland) as a result of thermal metamorphism influence. Arch. Min. Sci. 2010, 55, 783–798. [Google Scholar]
- UNECE International Classification of In-Seam Coals; UNECE Geneva: New York, NY, USA, 1998; p. 41.
- Zhou, Y.; Zhao, J. Advances in Rock Dynamics and Applications; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Pan, Z.; Connell, L.D. A theoretical model for gas adsorption-induced coal swelling. Int. J. Coal Geol. 2007, 69, 243–252. [Google Scholar] [CrossRef]
- Chareonsuppanimit, P.; Mohammad, S.A.; Robinson, R.L.; Gasem, K.A.M. Modeling gasadsorption-induced swelling and permeability changes in coals. Int. J. Coal Geol. 2014, 121, 98–109. [Google Scholar] [CrossRef]
- Wang, K.; Du, F.; Wang, G. Investigation of gas pressure and temperature effects on the permeability and steady-state time of chinese anthracite coal: An experimental study. J. Nat. Gas Sci. Eng. 2017, 40, 179–188. [Google Scholar] [CrossRef]
- Harpalani, S.; Chen, G. Influence of gas production induced volumetric strain on permeability of coal. Geotech. Geol. Eng. 1997, 15, 303–325. [Google Scholar] [CrossRef]
- Ma, Q.; Harpalani, S.; Liu, S. A simplified permeability model for coalbed methane reservoirs based on matchstick strain and constant volume theory. Int. J. Coal Geol. 2011, 85, 43–48. [Google Scholar] [CrossRef]
- Kumar, H.; Elsworth, D.; Liu, J.; Pone, D.; Mathews, J.P. Optimizing enhanced coalbed methane recovery for unhindered production and CO2 injectivity. Int. J. Greenh. Gas Control 2012, 11, 86–97. [Google Scholar] [CrossRef]
- Ma, D.; Miao, X.; Wu, Y.; Bai, H.; Wang, J.; Rezania, M.; Huang, Y.; Qian, H. Seepage properties of crushed coal particles. J. Pet. Sci. Eng. 2016, 146, 297–307. [Google Scholar]
- Wu, Y.; Pruess, K.; Persoff, P. Gas Flow in Porous Media with Klinkenberg Effects. Transp. Porous Media 1998, 32, 117–137. [Google Scholar] [CrossRef]
- Majewska, Z.; Majewski, S.; Ziętek, J. Swelling of coal induced by cyclic sorption/desorption of gas: Experimental observations indicating changes in coal structure due to sorption of CO2 and CH4. Int. J. Coal Geol. 2010, 83, 475–483. [Google Scholar] [CrossRef]
- Baran, P.; Zarębska, K.; Bukowska, M. Expansion of hard coal accompanying the sorption of methane and carbon dioxide in isothermal and non-isothermal processes. Energy Fuels 2015, 29, 1899–1904. [Google Scholar] [CrossRef]









| Physical Properties | Petrographic Analysis * | |||||
|---|---|---|---|---|---|---|
| ρe (g/cm3) | ρsk (g/cm3) | ϕ (%) | Ro (%) | Vitrinite (%) | Inertinite (%) | Liptinite (%) |
| 1.203 | 1.392 | 13.6 | 1.29 | 65.3 | 33.5 | 1.2 |
| Sample Symbol | Sample Pressing Pressure (MPa) | Slenderness Ratio (length/diameter) | Initial Porosity (%) | Type of Gas Used in Permeability Tests |
|---|---|---|---|---|
| N1 | 47.9 | 0.64 | 18.87 | Nitrogen |
| N2 | 71.9 | 0.62 | 15.81 | |
| N3 | 83.9 | 0.60 | 14.24 | |
| N4 | 95.8 | 0.59 | 12.91 | |
| M1 | 83.9 | 0.62 | 14.41 | Methane |
| M2 | 119.8 | 0.60 | 11.33 | |
| M3 | 155.7 | 0.59 | 9.97 | |
| M4 | 191.7 | 0.58 | 9.21 |
| Sample | Stress (MPa) | Porosity (%) | Permeability Coefficient k∞ (mD) | Slippage Factor b (MPa) |
|---|---|---|---|---|
| N1 | 0.00 | 18.87 | 0.816 | 0.072 |
| 6.26 | 18.24 | 0.611 | 0.061 | |
| 12.12 | 17.72 | 0.492 | 0.076 | |
| 18.27 | 17.24 | 0.428 | 0.078 | |
| 24.40 | 16.83 | 0.366 | 0.093 | |
| 30.10 | 16.52 | 0.320 | 0.106 | |
| N2 | 0.00 | 15.81 | 0.249 | 0.122 |
| 5.99 | 15.23 | 0.175 | 0.136 | |
| 12.30 | 14.71 | 0.132 | 0.152 | |
| 18.10 | 14.32 | 0.109 | 0.170 | |
| 24.54 | 13.99 | 0.098 | 0.159 | |
| 30.22 | 13.78 | 0.080 | 0.195 | |
| N3 | 0.00 | 14.24 | 0.142 | 0.163 |
| 6.06 | 13.78 | 0.106 | 0.165 | |
| 12.14 | 13.38 | 0.082 | 0.177 | |
| 18.17 | 13.07 | 0.069 | 0.170 | |
| 24.01 | 12.84 | 0.059 | 0.180 | |
| 30.30 | 12.67 | 0.050 | 0.195 | |
| N4 | 0.00 | 12.91 | 0.083 | 0.187 |
| 5.99 | 12.48 | 0.063 | 0.173 | |
| 11.98 | 12.11 | 0.039 | 0.260 | |
| 17.97 | 11.81 | 0.030 | 0.291 | |
| 23.96 | 11.58 | 0.023 | 0.345 | |
| 29.95 | 11.42 | 0.021 | 0.324 |
| Sample | Stress (MPa) | Porosity (%) | Permeability Coefficient k∞ (mD) | Slippage Factor b (MPa) |
|---|---|---|---|---|
| M1 | 0.00 | 14.41 | 0.01031 | 0.171 |
| 6.01 | 14.02 | 0.00835 | 0.188 | |
| 12.05 | 13.69 | 0.00755 | 0.185 | |
| 18.25 | 13.41 | 0.00713 | 0.181 | |
| 24.23 | 13.18 | 0.00686 | 0.177 | |
| 30.11 | 12.99 | 0.00649 | 0.174 | |
| M2 | 0.00 | 11.33 | 0.00316 | 0.264 |
| 6.11 | 10.87 | 0.00291 | 0.211 | |
| 12.15 | 10.48 | 0.00251 | 0.198 | |
| 18.07 | 10.16 | 0.00210 | 0.204 | |
| 23.98 | 9.89 | 0.00178 | 0.212 | |
| 29.90 | 9.75 | 0.00154 | 0.205 | |
| M3 | 0.00 | 9.97 | 0.00195 | 0.290 |
| 6.02 | 9.61 | 0.00150 | 0.295 | |
| 12.12 | 9.29 | 0.00120 | 0.293 | |
| 17.95 | 9.02 | 0.00104 | 0.273 | |
| 23.97 | 8.79 | 0.00096 | 0.221 | |
| 29.89 | 8.55 | 0.00084 | 0.228 | |
| M4 | 0.00 | 9.21 | 0.00127 | 0.265 |
| 5.98 | 8.76 | 0.00089 | 0.298 | |
| 12.11 | 8.37 | 0.00082 | 0.254 | |
| 18.03 | 8.04 | 0.00065 | 0.272 | |
| 24.08 | 7.77 | 0.00055 | 0.254 | |
| 29.92 | 7.41 | 0.00052 | 0.267 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudasik, M. Investigating Permeability of Coal Samples of Various Porosities under Stress Conditions. Energies 2019, 12, 762. https://0-doi-org.brum.beds.ac.uk/10.3390/en12040762
Kudasik M. Investigating Permeability of Coal Samples of Various Porosities under Stress Conditions. Energies. 2019; 12(4):762. https://0-doi-org.brum.beds.ac.uk/10.3390/en12040762
Chicago/Turabian StyleKudasik, Mateusz. 2019. "Investigating Permeability of Coal Samples of Various Porosities under Stress Conditions" Energies 12, no. 4: 762. https://0-doi-org.brum.beds.ac.uk/10.3390/en12040762