Experimental Investigation on the Energy Consumption, Physical, and Thermal Properties of a Novel Pellet Fuel Made from Wood Residues with Microalgae as a Binder
Abstract
:1. Introduction
2. Materials and Methods
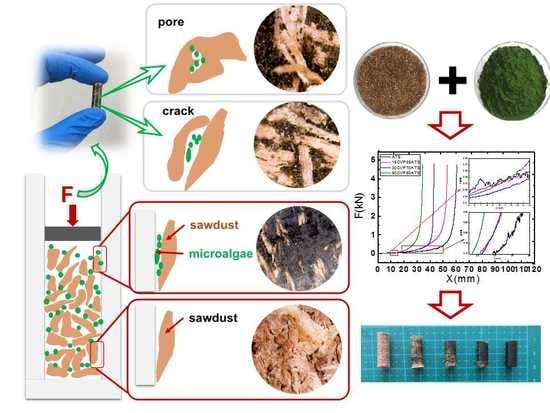
2.1. Materials
2.2. Pelletization and Sample Preparation
2.3. Evaluating Properties of Pellets (Based on ISO 17225)
2.4. The Porosity (ϕ0) of Pellets
2.5. Energy Consumption of Pelletization
2.6. Thermogravimetric Experiments
2.7. Kinetic Model
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effects of Parameters on the Physical Properties of CSBs
3.1.1. Effect of Pressure on Physical Properties
3.1.2. Effect of Temperature on Physical Properties
3.1.3. Effect of Moisture Content on Physical Properties
3.2. Insight into the Energy Consumption of CSBs During Co-Pelletization
3.3. Analysis of the Potential of Microalgae Compared with Other Binders
3.4. Thermal Characterization of CSBs
3.5. Influence of Heating Rate on the Co-Combustion of CSBs
3.6. Thermal Characterization of Interaction Between CVP and ATS
3.7. Kinetic Analysis of CSBs
3.8. Energy Efficiency Perspective of the Pellet
4. Conclusions
- 1)
- Increasing the temperature (80–160 °C) and pressure (120–200 MPa) could effectively increase the BD and DU of the novel pellets and blending microalgae could effectively increase the physical properties of the pellets. Moreover, the moisture content had an optimal value for the physical properties of the novel pellets. Blending microalgae could significantly reduce the energy consumption of pelleting by 23.5%–40.4%. Moreover, when the amount of CVP was 50%, a maximum BD of 1580.2 kg/m3, a DU of 98%, and a minimum energy consumption of 25.2 kJ/kg were obtained under the optimum conditions of temperature (120 °C), pressure (120 MPa), and moisture content (10%), respectively.
- 2)
- The combustion experiment showed that the co-combustion interaction between the CVP and ATS did exist, and their effect on co-combustion process was inhibitive (0–300 °C) and accelerative (300–780 °C). Blending CVP could increase the Mf, Tf, and DTGmax and decrease T2, ICC, and Ti.
- 3)
- The kinetic analysis showed that the minimum average E value of a pellet was obtained when the blending ratio of microalgae was 15%, which was 133.21 kJ/mol and 134.60 kJ/mol calculated by the KAS and OFW methods, respectively. Therefore, considering the co-pelletization and co-combustion of CSBs, blending 15% of microalgae could be considered an excellent way to improve the properties of CSBs.
- 4)
- Thus, the quality of a bio-pellet like ATS could effectively be improved by using microalgae as a binder. The novel bio-pellet fuel made from waste biomass and microalgae blends is a promising option for biofuel production.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| List of Abbreviations: | |||
| TGA | Thermogravimetric analysis | ATS | Apple tree sawdust |
| CVP | Chlorella vulgaris powder | 15CVP85ATS | Composed of 15% CVP and 85% ATS |
| KAS | Kissinger–Akahira–Sunose method | 30CVP70ATS | Composed of 30% CVP and 70% ATS |
| OFW | Ozawa–Flynn–Wall method | 50CVP50ATS | Composed of 50% CVP and 50% ATS |
| CSB | Chlorella vulgaris and sawdust blend | LHV | the low heating value |
| HHV | the high heating value | ||
| List of Symbols: | |||
| V | Volatile matters | T | Absolute reaction temperature (°C) |
| Fc | Fixed carbon | β | Heating rate (°C/min) |
| A | Ash | TG | Thermogravimetric data (%) |
| BD | Bulk density (kg/m3 Wb) | DTG | First derivative data of TG (%/sec) |
| DU | Mechanical durability (%) | CCI | Comprehensive combustion index |
| W | Energy consumption (kJ/kg) | DTGmax | Maximum mass loss rate (%/sec) |
| F | Pressing force (kN) | DTGmean | Average mass loss rate (%/sec) |
| X | Displacement (mm) | Ti | Ignition temperature (°C) |
| α | Conversion | Tf | Burnout temperature (°C) |
| mi | Initial weights (mg) | Mf | Residual mass (%) |
| mt | Instantaneous weights (mg) | T1 | Temperature of the first peak (°C) |
| m∞ | Final weights (mg) | T2 | Temperature of the second peak (°C) |
| k | Rate constant | Wexperimental | Weight loss values experimental (%) |
| A | Pre-exponential factor | Wcalculated | Weight loss values calculated (%) |
| E | Activation energy of the reaction | xCVP | Proportion of CVP (%) |
| R | Constant, 8.314 J/ (mol·K) | xATS | Proportion of ATS (%) |
| ϕ0 | Porosity of pellet (%) | ΔW | Degree of interaction |
Appendix A
| Samples | α | KAS Method | OFW Method | ||
|---|---|---|---|---|---|
| E (kJ/mol) | R2 | E (kJ/mol) | R2 | ||
| ATS | 0.2 | 115.08 | 0.99918 | 120.02 | 0.99941 |
| 0.3 | 131.83 | 0.99974 | 136.41 | 0.99948 | |
| 0.4 | 142.63 | 0.89562 | 146.93 | 0.91083 | |
| 0.5 | 150.87 | 0.87344 | 146.93 | 0.89874 | |
| 0.6 | 160.17 | 0.8922 | 159.39 | 0.91083 | |
| 0.7 | 141.22 | 0.9215 | 139.59 | 0.93359 | |
| 0.8 | 122.17 | 0.9969 | 121.34 | 0.99773 | |
| Average | 137.71 | - | 138.66 | - | |
| 15CVP85ATS | 0.2 | 110.49 | 0.99313 | 113.48 | 0.90887 |
| 0.3 | 118.31 | 0.92862 | 120.55 | 0.93547 | |
| 0.4 | 131.84 | 0.98243 | 132.56 | 0.98422 | |
| 0.5 | 136.11 | 0.95988 | 137.32 | 0.96446 | |
| 0.6 | 168.03 | 0.92623 | 168.7 | 0.99607 | |
| 0.7 | 144.5 | 0.91053 | 149.36 | 0.98132 | |
| 0.8 | 123.2 | 0.9062 | 120.2 | 0.97536 | |
| Average | 133.21 | - | 134.60 | - | |
| 30CVP70ATS | 0.2 | 105.23 | 0.97168 | 113.43 | 0.99964 |
| 0.3 | 116.98 | 0.97362 | 124.38 | 0.9998 | |
| 0.4 | 126.68 | 0.921 | 128.18 | 0.95306 | |
| 0.5 | 147.7 | 0.98045 | 141.51 | 0.91316 | |
| 0.6 | 181.26 | 0.98139 | 183.09 | 0.93694 | |
| 0.7 | 165.49 | 0.92352 | 162.07 | 0.9294 | |
| 0.8 | 134.8 | 0.92318 | 137.07 | 0.98387 | |
| Average | 139.73 | - | 141.39 | - | |
| 50CVP50ATS | 0.2 | 96.71 | 0.93247 | 99.06 | 0.97914 |
| 0.3 | 100.65 | 0.94912 | 110.15 | 0.92945 | |
| 0.4 | 111.31 | 0.98741 | 106.06 | 0.97082 | |
| 0.5 | 153.88 | 0.9903 | 155.96 | 0.9833 | |
| 0.6 | 174.8 | 0.98008 | 181.05 | 0.91383 | |
| 0.7 | 195.42 | 0.95383 | 198.12 | 0.98519 | |
| 0.8 | 150.32 | 0.96674 | 153.86 | 0.97334 | |
| Average | 140.44 | - | 143.47 | - | |
| CVP | 0.2 | 71.24 | 0.90621 | 70.44 | 0.90621 |
| 0.3 | 93.46 | 0.94546 | 95.84 | 0.94546 | |
| 0.4 | 118.58 | 0.99395 | 115.32 | 0.99395 | |
| 0.5 | 148.94 | 0.94908 | 149.35 | 0.94908 | |
| 0.6 | 177.88 | 0.93399 | 172.56 | 0.93399 | |
| 0.7 | 209.36 | 0.98784 | 211.06 | 0.98784 | |
| 0.8 | 180.58 | 0.94417 | 179.60 | 0.96417 | |
| Average | 142.86 | - | 142.02 | - | |
References
- Raheem, A.; Prinsen, P.; Vuppaladadiyam, A.K.; Zhao, M.; Luque, R. A review on sustainable microalgae based biofuel and bioenergy production: Recent developments. J. Clean. Prod. 2018, 181, 42–59. [Google Scholar] [CrossRef]
- Park, C.; Heo, K.; Oh, S.; Kim, S.B.; Lee, S.H.; Kim, Y.H.; Kim, Y.; Lee, J.; Han, S.O.; Lee, S.; et al. Eco-design and evaluation for production of 7-aminocephalosporanic acid from carbohydrate wastes discharged after microalgae-based biodiesel production. J. Clean. Prod. 2016, 133, 511–517. [Google Scholar] [CrossRef]
- Ou, L.; Thilakaratne, R.; Brown, R.C.; Wright, M.M. Techno-economic analysis of transportation fuels from defatted microalgae via hydrothermal liquefaction and hydroprocessing. Biomass Bioenergy 2015, 72, 45–54. [Google Scholar] [CrossRef]
- Hess, D.; Quinn, J.C. Impact of inorganic contaminants on microalgal biofuel production through multiple conversion pathways. Biomass Bioenergy 2018, 119, 237–245. [Google Scholar] [CrossRef]
- Hosseinizand, H.; Sokhansanj, S.; Lim, C.J. Co-pelletization of microalgae Chlorella vulgaris and pine sawdust to produce solid fuels. Fuel Process. Technol. 2018, 177, 129–139. [Google Scholar] [CrossRef]
- Krizan, P.; Matus, M.; Soos, L.; Beniak, J. Behavior of Beech Sawdust during Densification into a Solid Biofuel. Energies 2015, 8, 6382–6398. [Google Scholar] [CrossRef] [Green Version]
- Amarasekara, A.; Tanzim, F.S.; Asmatulu, E. Briquetting and carbonization of naturally grown algae biomass for low-cost fuel and activated carbon production. Fuel 2017, 208, 612–617. [Google Scholar] [CrossRef]
- Miranda, M.T.; Sepulveda, F.J.; Arranz, J.I.; Montero, I.; Rojas, C.V. Physical-energy characterization of microalgae Scenedesmus and experimental pellets. Fuel 2018, 226, 121–126. [Google Scholar] [CrossRef]
- Cui, X.; Yang, J.; Lei, W.; Huang, T.; Wang, P.; Jia, C. Recent progress in research and application of DBBF additive in preparation and combustion process. Huagong Jinzhan/Chem. Ind. Eng. Prog. 2017, 36, 1247–1257. [Google Scholar]
- Muazu, R.I.; Stegemann, J.A. Biosolids and microalgae as alternative binders for biomass fuel briquetting. Fuel 2017, 194, 339–347. [Google Scholar] [CrossRef]
- Guo, F.; Zhong, Z. Optimization of the co-combustion of coal and composite biomass pellets. J. Clean. Prod. 2018, 185, 399–407. [Google Scholar] [CrossRef]
- Chen, C.; Ma, X.; He, Y. Co-pyrolysis characteristics of microalgae Chlorella vulgaris and coal through TGA. Bioresour. Technol. 2012, 117, 264–273. [Google Scholar] [CrossRef]
- Peng, X.; Ma, X.; Xu, Z. Thermogravimetric analysis of co-combustion between microalgae and textile dyeing sludge. Bioresour. Technol. 2015, 180, 288–295. [Google Scholar] [CrossRef]
- Arteaga-Perez, L.E.; Vega, M.; Rodriguez, L.C.; Flores, M.; Zaror, C.A.; Casas Ledon, Y. Life-Cycle Assessment of coal-biomass based electricity in Chile: Focus on using raw vs torrefied wood. Energy Sustain. Dev. 2015, 29, 81–90. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Li, H.; Chen, X.; Xiao, Z.; Liang, J.; Leng, L.; Guo, Z.; Zeng, G. Co-pelletization of sewage sludge and biomass: Thermogravimetric analysis and ash deposits. Fuel Process. Technol. 2016, 145, 109–115. [Google Scholar] [CrossRef]
- Magdziarz, A.; Gajek, M.; Nowak-Wozny, D.; Wilk, M. Mineral phase transformation of biomass ashes—Experimental and thermochemical calculations. Renew. Energy 2018, 128, 446–459. [Google Scholar] [CrossRef]
- Rybak, W.; Moron, W.; Ferens, W. Dust ignition characteristics of different coal ranks, biomass and solid waste. Fuel 2019, 237, 606–618. [Google Scholar] [CrossRef]
- Chen, G.; Wang, X.; Li, J.; Yan, B.; Wang, Y.; Wu, X.; Velichkova, R.; Cheng, Z.; Ma, W. Environmental, energy, and economic analysis of integrated treatment of municipal solid waste and sewage sludge: A case study in China. Sci. Total Environ. 2019, 647, 1433–1443. [Google Scholar] [CrossRef]
- Xiao, Z.; Yuan, X.; Jiang, L.; Chen, X.; Li, H.; Zeng, G.; Leng, L.; Wang, H.; Huang, H. Energy recovery and secondary pollutant emission from the combustion of co-pelletized fuel from municipal sewage sludge and wood sawdust. Energy 2015, 91, 441–450. [Google Scholar] [CrossRef]
- Yilmaz, E.; Wzorek, M.; Akcay, S. Co-pelletization of sewage sludge and agricultural wastes. J. Environ. Manag. 2018, 216, 169–175. [Google Scholar] [CrossRef]
- Tumuluru, J.S. Pelleting of Pine and Switchgrass Blends: Effect of Process Variables and Blend Ratio on the Pellet Quality and Energy Consumption. Energies 2019, 12, 11987. [Google Scholar] [CrossRef]
- Asadieraghi, M.; Daud, W.M.A.W. In-depth investigation on thermochemical characteristics of palm oil biomasses as potential biofuel sources. J. Anal. Appl. Pyrolysis 2015, 115, 379–391. [Google Scholar] [CrossRef]
- Cao, L.; Yuan, X.; Jiang, L.; Li, C.; Xiao, Z.; Huang, Z.; Chen, X.; Zeng, G.; Li, H. Thermogravimetric characteristics and kinetics analysis of oil cake and torrefied biomass blends. Fuel 2016, 175, 129–136. [Google Scholar] [CrossRef]
- ISO. ISO 18134-1/2 Solid Biofuels—Determination of Moisture Content—Oven Dry Method—Part 1/2; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- ISO. ISO 18122, Solid Biofuels. Determination of Ash Content; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- ISO. ISO 18125, Solid Biofuels—Determination of Calorific Value; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- ISO. ISO 17831-1, Solid Biofuels. Determination of Mechanical Durability of Pellets and Briquettes; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- ISO. ISO 17828, Solid Biofuels—Determination of Bulk Density; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- Song, X.; Yang, Y.; Zhang, M.; Zhang, K.; Wang, D. Ultrasonic pelleting of torrefied lignocellulosic biomass for bioenergy production. Renew. Energy 2018, 129, 56–62. [Google Scholar] [CrossRef]
- Li, G.; Liu, C.; Yu, Z.; Rao, M.; Zhong, Q.; Zhang, Y.; Jiang, T. Energy Saving of Composite Agglomeration Process (CAP) by Optimized Distribution of Pelletized Feed. Energies 2018, 11, 2382. [Google Scholar] [CrossRef]
- Tauro, R.; Serrano-Medrano, M.; Masera, O. Solid biofuels in Mexico: A sustainable alternative to satisfy the increasing demand for heat and power. Clean Technol. Environ. Policy 2018, 20, 1527–1539. [Google Scholar] [CrossRef]
- Paredes-Sanchez, J.P.; Lopez-Ochoa, L.M.; Lopez-Gonzalez, L.M.; Las-Heras-Casas, J.; Xiberta-Bernat, J. Energy utilization for distributed thermal production in rural areas: A case study of a self-sustaining system in Spain. Energy Convers. Manag. 2018, 174, 1014–1023. [Google Scholar] [CrossRef]
- Pereira, M.F.; Nicolau, V.P.; Bazzo, E. Exergoenvironmental analysis concerning the wood chips and wood pellets production chains. Biomass Bioenergy 2018, 119, 253–262. [Google Scholar] [CrossRef]
- Sun, Y.; Bai, F.; Lu, X.; Jia, C.; Wang, Q.; Guo, M.; Li, Q.; Guo, W. Kinetic study of Huadian oil shale combustion using a multi-stage parallel reaction model. Energy 2015, 82, 705–713. [Google Scholar] [CrossRef]
- Lopez, R.; Fernandez, C.; Fierro, J.; Cara, J.; Martinez, O.; Sanchez, M.E. Oxy-combustion of corn, sunflower, rape and microalgae bioresidues and their blends from the perspective of thermogravimetric analysis. Energy 2014, 74, 845–854. [Google Scholar] [CrossRef]
- Hernández, D.; Solana, M.; Riaño, B.; García-González, M.C.; Bertucco, A. Biofuels from microalgae: Lipid extraction and methane production from the residual biomass in a biorefinery approach. Bioresour. Technol. 2014, 170, 370–378. [Google Scholar] [CrossRef]
- Timmons, D.; Mejía, C.V. Biomass energy from wood chips: Diesel fuel dependence? Biomass Bioenergy 2010, 34, 1419–1425. [Google Scholar] [CrossRef]
- Faizal, H.M.; Shamsuddin, H.S.; Heiree, M.H.M.; Hanaffi, M.F.M.A.; Rahman, M.R.A.; Rahman, M.M.; Latiff, Z.A. Torrefaction of densified mesocarp fibre and palm kernel shell. Renew. Energy 2018, 122, 419–428. [Google Scholar] [CrossRef]
- Jiang, L.; Liang, J.; Yuan, X.; Li, H.; Li, C.; Xiao, Z.; Huang, H.; Wang, H.; Zeng, G. Co-pelletization of sewage sludge and biomass: The density and hardness of pellet. Bioresour. Technol. 2014, 166, 435–443. [Google Scholar] [CrossRef]
- Kaliyan, N.; Morey, R.V. Natural binders and solid bridge type binding mechanisms in briquettes and pellets made from corn stover and switchgrass. Bioresour. Technol. 2010, 101, 1082–1090. [Google Scholar] [CrossRef]
- Kaliyan, N.; Morey, R.V.; Schmidt, D.R. Roll press compaction of corn stover and perennial grasses to increase bulk density. Biomass Bioenergy 2013, 55, 322–330. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Xiao, Z.; Liang, J.; Li, H.; Cao, L.; Wang, H.; Chen, X.; Zeng, G. A comparative study of biomass pellet and biomass-sludge mixed pellet: Energy input and pellet properties. Energy Convers. Manag. 2016, 126, 509–515. [Google Scholar] [CrossRef]
- Alberto Garcia-Nunez, J.; Tatiana Rodriguez, D.; Andres Fontanilla, C.; Elizabeth Ramirez, N.; Silva Lora, E.E.; Frear, C.S.; Stockle, C.; Amonette, J.; Garcia-Perez, M. Evaluation of alternatives for the evolution of palm oil mills into biorefineries. Biomass Bioenergy 2016, 95, 310–329. [Google Scholar] [CrossRef]
- Kaliyan, N.; Morey, R.V. Densification characteristics of corn cobs. Fuel Process. Technol. 2010, 91, 559–565. [Google Scholar] [CrossRef]
- Kaliyan, N.; Morey, R.V. Constitutive model for densification of corn stover and switchgrass. Biosyst. Eng. 2009, 104, 47–63. [Google Scholar] [CrossRef]
- Bai, X.; Wang, G.; Gong, C.; Yu, Y.; Liu, W.; Wang, D. Co-pelletizing characteristics of torrefied wheat straw with peanut shell. Bioresour. Technol. 2017, 233, 373–381. [Google Scholar] [CrossRef]
- Cao, L.; Yuan, X.; Li, H.; Li, C.; Xiao, Z.; Jiang, L.; Huang, B.; Xiao, Z.; Chen, X.; Wang, H.; et al. Complementary effects of torrefaction and co-pelletization: Energy consumption and characteristics of pellets. Bioresour. Technol. 2015, 185, 254–262. [Google Scholar] [CrossRef]
- Ahn, B.J.; Chang, H.; Lee, S.M.; Choi, D.H.; Cho, S.T.; Han, G.; Yang, I. Effect of binders on the durability of wood pellets fabricated from Larix kaemferi C. and Liriodendron tulipifera L. sawdust. Renew. Energy 2014, 62, 18–23. [Google Scholar] [CrossRef]
- Si, Y.; Hu, J.; Wang, X.; Yang, H.; Chen, Y.; Shao, J.; Chen, H. Effect of Carboxymethyl Cellulose Binder on the Quality of Biomass Pellets. Energy Fuels 2016, 30, 5799–5808. [Google Scholar] [CrossRef]
- Berghel, J.; Frodeson, S.; Granstrom, K.; Renstrom, R.; Stahl, M.; Nordgren, D.; Tomani, P. The effects of kraft lignin additives on wood fuel pellet quality, energy use and shelf life. Fuel Process. Technol. 2013, 112, 64–69. [Google Scholar] [CrossRef]
- Hu, Q.; Shao, J.; Yang, H.; Yao, D.; Wang, X.; Chen, H. Effects of binders on the properties of bio-char pellets. Appl. Energy 2015, 157, 508–516. [Google Scholar] [CrossRef]
- Li, H.; Jiang, L.; Li, C.; Liang, J.; Yuan, X.; Xiao, Z.; Xiao, Z.; Wang, H. Co-pelletization of sewage sludge and biomass: The energy input and properties of pellets. Fuel Process. Technol. 2015, 132, 55–61. [Google Scholar] [CrossRef]
- Holm, J.K.; Henriksen, U.B.; Hustad, J.E.; Sorensen, L.H. Toward an understanding of controlling parameters in softwood and hardwood pellets production. Energy Fuels 2006, 20, 2686–2694. [Google Scholar] [CrossRef]
- Frodeson, S.; Henriksson, G.; Berghel, J. Pelletizing Pure Biomass Substances to Investigate the Mechanical Properties and Bonding Mechanisms. BioResources 2018, 13, 1202–1222. [Google Scholar] [CrossRef]
- Masche, M.; Puig-Arnavat, M.; Jensen, P.A.; Holm, J.K.; Clausen, S.; Ahrenfeldt, J.; Henriksen, U.B. From wood chips to pellets to milled pellets: The mechanical processing pathway of Austrian pine and European beech. Powder Technol. 2019, 350, 134–145. [Google Scholar] [CrossRef]
- Marrugo, G.; Valdes, C.F.; Gomez, C.; Chejne, F. Pelletizing of Colombian agro-industrial biomasses with crude glycerol. Renew. Energy 2019, 134, 558–568. [Google Scholar] [CrossRef]
- Azargohar, R.; Nanda, S.; Kang, K.; Bond, T.; Karunakaran, C.; Dalai, A.K.; Kozinski, J.A. Effects of bio-additives on the physicochemical properties and mechanical behavior of canola hull fuel pellets. Renew. Energy 2019, 132, 296–307. [Google Scholar] [CrossRef]
- Lawoko, M.; Berggren, R.; Berthold, F.; Henriksson, G.; Gellerstedt, G. Changes in the lignin-carbohydrate complex in softwood kraft pulp during kraft and oxygen delignification. Holzforschung 2004, 58, 603–610. [Google Scholar] [CrossRef]
- Dai, X.; Theppitak, S.; Yoshikawa, K. Pelletization of Carbonized Wood Using Organic Binders with Biomass Gasification Residue as an Additive. Energy Fuels 2019, 33, 323–329. [Google Scholar] [CrossRef]
- Winzer, F.; Kraska, T.; Elsenberger, C.; Koetter, T.; Pude, R. Biomass from fruit trees for combined energy and food production. Biomass Bioenergy 2017, 107, 279–286. [Google Scholar] [CrossRef]
- Yuan, T.; Tahmasebi, A.; Yu, J. Comparative study on pyrolysis of lignocellulosic and algal biomass using a thermogravimetric and a fixed-bed reactor. Bioresour. Technol. 2015, 175, 333–341. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Kassim, M.A.; Yu, J.; Bhattacharya, S. Thermogravimetric study of the combustion of Tetraselmis suecica microalgae and its blend with a Victorian brown coal in O-2/N-2 and O-2/CO2 atmospheres. Bioresour. Technol. 2013, 150, 15–27. [Google Scholar] [CrossRef]
- Huang, Z.L.; Li, H.; Yuan, X.Z.; Lin, L.; Cao, L.; Xiao, Z.H.; Jiang, L.B.; Li, C.Z. The energy consumption and pellets’ characteristics in the co-pelletization of oil cake and sawdust. RSC Adv. 2016, 6, 19199–19207. [Google Scholar] [CrossRef]
- Rezaei, H.; Yazdanpanah, F.; Lim, C.J.; Lau, A.; Sokhansanj, S. Pyrolysis of ground pine chip and ground pellet particles. Can. J. Chem. Eng. 2016, 94, 1863–1871. [Google Scholar] [CrossRef]
- Rezaei, H.; Sokhansanj, S.; Lim, C.J. Minimum fluidization velocity of ground chip and ground pellet particles of woody biomass. Chem. Eng. Process. 2018, 124, 222–234. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Tomáš, O.; Ivan, V.; Tomáš, H. Non-isothermal kinetic analysis of the thermal decomposition of spruce wood in air atmosphere. Res. Agric. Eng. 2018, 64, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Orfão, J.J.; Antunes, F.J.; Figueiredo, J.L. Pyrolysis kinetics of lignocellulosic materials—Three independent reactions model. Fuel 1999, 78, 349–358. [Google Scholar] [CrossRef]
- Adams, P.W.R.; Shirley, J.E.J.; Mcmanus, M.C. Comparative cradle-to-gate life cycle assessment of wood pellet production with torrefaction. Appl. Energy 2015, 138, 367–380. [Google Scholar] [CrossRef] [Green Version]
- Lazaroiu, G.; Mihaescu, L.; Negreanu, G.; Pana, C.; Pisa, I.; Cernat, A.; Ciupageanu, D. Experimental Investigations of Innovative Biomass Energy Harnessing Solutions. Energies 2018, 11, 3469. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Lam, M.K.; Yusup, S.; Lim, J.W.; Lee, K.T. Life cycle evaluation of microalgae biofuels production: Effect of cultivation system on energy, carbon emission and cost balance analysis. Sci. Total Environ. 2019, 688, 112–128. [Google Scholar] [CrossRef]
- Lazaroiu, G.; Mihăescu, L.; Prisecaru, T.; Oprea, I.; Pîşă, I.; Negreanu, G.; Indrieş, R.; Popa, V.I. Combustion of pitcoal-wood biomass brichettes in a boiler test facility. Environ. Eng. Manag. J. 2008, 7, 595–601. [Google Scholar] [CrossRef]
- Lazaroiu, G.; Oprea, I.; Mihaescu, L.; Prisecaru, T.; Pisa, I.; Negreanu, G.; Mocanu, C.R. Biomass briquettes from pitcoal-wood: Boiler test facility combustion case study. J. Environ. Prot. Ecol. 2012, 13, 1070–1081. [Google Scholar]
- Lazaroiu, G.; Pop, E.; Negreanu, G.; Pisa, I.; Mihaescu, L.; Bondrea, A.; Berbece, V. Biomass combustion with hydrogen injection for energy applications. Energy 2017, 127, 351–357. [Google Scholar] [CrossRef]
- Neri, E.; Cespi, D.; Setti, L.; Gombi, E.; Bernardi, E.; Vassura, I.; Passarini, F. Biomass residues to renewable energy: A life cycle perspective applied at a local scale. Energies 2016, 9, 922. [Google Scholar] [CrossRef]
- Katers, J.F.; Snippen, A.J.; Puettmann, M.E. Life-cycle inventory of wood pellet manufacturing and utilization in Wisconsin. For. Prod. J. 2012, 62, 289–295. [Google Scholar] [CrossRef]













| Properties | Apple Tree Sawdust | Microalgae (Chlorella vulgaris) |
|---|---|---|
| Proximate analysis (%) | ||
| V | 77.08 ± 0.71 | 79.76 ± 0.62 |
| A | 5.30 ± 0.16 | 6.81 ± 0.21 |
| Fc | 17.62 ± 1.24 | 13.43 ± 0.81 |
| Ultimate analysis (%) | ||
| C | 45.32 ± 0.02 | 49.13 ± 0.12 |
| H | 5.635 ± 0.21 | 7.26 ± 0.11 |
| O | 48.11 ± 0.14 | 33.96 ± 0.16 |
| N | 0.89 ± 0.03 | 9.12 ± 0.08 |
| S | 0.044 ± 0.01 | 0.53 ± 0.05 |
| LHV (MJ/kg) | 10.01 ± 0.12 | 11.01 ± 0.02 |
| HHV (MJ/kg) | 17.41 ± 0.42 | 21.90 ± 0.47 |
| Macromolecular analysis (%) | ||
| Cellulose | 42.83 ± 0.12 | - |
| Hemicellulose | 25.37 ± 0.12 | - |
| Lignin | 25.78 ± 0.12 | - |
| Lipid | - | 10.66 ± 0.12 |
| Protein | - | 11.61 ± 0.12 |
| Carbohydrate (Polymer) | - | 26.23 ± 0.12 |
| Sample | BD (kg/m3) | Error | DU (%) | Error | W (kJ/kg) | Error |
|---|---|---|---|---|---|---|
| ATS | 1161.4 | ±5.3% | 96.6 | ±1.3% | 42.1 | ±3.3% |
| 15CVP85ATS | 1277.3 | ±6.1% | 97.3 | ±1.9% | 32.2 | ±3.1% |
| 30CVP70ATS | 1345.3 | ±4.4% | 97.8 | ±1.4% | 28.3 | ±4.7% |
| 50CVP50ATS | 1580.2 | ±5.2% | 98.3 | ±2.2% | 25.2 | ±4.2% |
| Heating Rate (°C/min) | Ti (°C) | Tf (°C) | T1 (°C) | T2 (°C) | DTGmax (%/sec) | Mf (%) |
|---|---|---|---|---|---|---|
| 20 | 271.1 | 674.4 | 83.4 | 321.7 | −0.574 | 10.78 |
| 30 | 272.3 | 668.9 | 85.3 | 327.2 | −0.642 | 9.81 |
| 40 | 280.5 | 664.6 | 85.9 | 329.2 | −0.668 | 8.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Yang, J.; Shi, X.; Lei, W.; Huang, T.; Bai, C. Experimental Investigation on the Energy Consumption, Physical, and Thermal Properties of a Novel Pellet Fuel Made from Wood Residues with Microalgae as a Binder. Energies 2019, 12, 3425. https://0-doi-org.brum.beds.ac.uk/10.3390/en12183425
Cui X, Yang J, Shi X, Lei W, Huang T, Bai C. Experimental Investigation on the Energy Consumption, Physical, and Thermal Properties of a Novel Pellet Fuel Made from Wood Residues with Microalgae as a Binder. Energies. 2019; 12(18):3425. https://0-doi-org.brum.beds.ac.uk/10.3390/en12183425
Chicago/Turabian StyleCui, Xuyang, Junhong Yang, Xinyu Shi, Wanning Lei, Tao Huang, and Chao Bai. 2019. "Experimental Investigation on the Energy Consumption, Physical, and Thermal Properties of a Novel Pellet Fuel Made from Wood Residues with Microalgae as a Binder" Energies 12, no. 18: 3425. https://0-doi-org.brum.beds.ac.uk/10.3390/en12183425