Hydrogen Production from Methane Cracking in Dielectric Barrier Discharge Catalytic Plasma Reactor Using a Nanocatalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Ni/MgAl2O4
2.2. Materials Characterisation
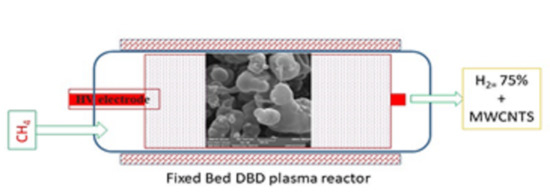
2.3. Plasma-Catalytic Methane Cracking System
3. Results and Discussion
3.1. Physicochemical Properties of the Catalyst
3.2. Plasma-Catalytic Methane Cracking
3.2.1. Plasma and Plasma-Catalytic Test and Reaction Mechanism
3.2.2. Time on-Stream Analysis of Ni/MgAl2O4
3.3. Characterisation of Spent Catalyst and Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goglio, R.; Smith, W.N.; Grant, B.B.; Desjardins, R.L.; Gao, X.; Hanis, K.; Tenuta, M.; Campbell, C.A.; McConkey, B.G.; Nemecek, T.; et al. A comparison of methods to quantify greenhouse gas emissions of cropping systems in LCA. J. Clean. Prod. 2018, 172, 4010–4017. [Google Scholar] [CrossRef] [Green Version]
- Salkuyeh, Y.K.; Adams, T.A. Combining coal gasification, natural gas reforming, and external carbonless heat for efficient production of gasoline and diesel with CO2 capture and sequestration. Energy Convers. Manag. 2013, 74, 492–504. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Zhang, S.; Sun, H.; Li, J.; Shao, T. Nanosecond pulsed plasma assisted dry reforming of CH4: The effect of plasma operating parameters. Appl. Energy 2019, 243, 132–144. [Google Scholar] [CrossRef]
- Schwietzke, S.; Sherwood, O.A.; Bruhwiler, L.M.; Miller, J.B.; Etiope, G.; Dlugokencky, E.J.; Michel, S.E.; Arling, V.A.; Vaughn, B.H.; White, J.W.; et al. Upward revision of global fossil fuel methane emissions based on isotope database. Nature 2016, 538, 88–91. [Google Scholar] [CrossRef]
- Razi, F.; Dincer, I. A critical evaluation of potential routes of solar hydrogen production for sustainable development. J. Clean. Prod. 2020, 264, 121582. [Google Scholar] [CrossRef]
- Khalifeh, O.; Taghvaei, H.; Mosallanejad, A.; Rahimpour, M.R.; Shariati, A. Extra pure hydrogen production through methane decomposition using nanosecond pulsed plasma and Pt–Re catalyst. Chem. Eng. J. 2016, 294, 132–145. [Google Scholar] [CrossRef]
- Zhang, R.; Cao, Y.; Li, H.; Zhao, Z.; Zhao, K.; Jiang, L. The role of CuO modified La0.7Sr0.3FeO3 perovskite on intermediate-temperature partial oxidation of methane via chemical looping scheme. Int. J. Hydrogen Energy 2020, 45, 4073–4083. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Recent developments in non-thermal catalytic DBD plasma reactor for dry reforming of methane. Energy Convers. Manag. 2019, 183, 529–560. [Google Scholar] [CrossRef]
- Khoja, A.H.; Anwar, M.; Shakir, S.; Mehran, M.T.; Mazhar, A.; Javed, A.; Amin, N.A.S. Thermal dry reforming of methane over La2O3 co-supported Ni/MgAl2O4 catalyst for hydrogen-rich syngas production. Res. Chem. Intermed. 2020, 46, 3817–3833. [Google Scholar] [CrossRef]
- Lašič Jurković, D.; Liu, J.-L.; Pohar, A.; Likozar, B. Methane Dry Reforming over Ni/Al2O3 Catalyst in Spark Plasma Reactor: Linking Computational Fluid Dynamics (CFD) with Reaction Kinetic Modelling. Catal. Today 2020. [Google Scholar] [CrossRef]
- Dan, M.; Mihet, M.; Lazar, M.D. Hydrogen and/or syngas production by combined steam and dry reforming of methane on nickel catalysts. Int. J. Hydrogen Energy 2020, 45, 26254–26264. [Google Scholar] [CrossRef]
- Snoeckx, R.; Bogaerts, A. Plasma technology—A novel solution for CO2 conversion? Chem. Soc. Rev. 2017, 46, 5805–5863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assad Munawar, M.; Hussain Khoja, A.; Hassan, M.; Liaquat, R.; Raza Naqvi, S.; Taqi Mehran, M.; Abdullah, A.; Saleem, F. Biomass ash characterization, fusion analysis and its application in catalytic decomposition of methane. Fuel 2021, 285, 119107. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Takriff, M.S. Methane decomposition into COx free hydrogen and multiwalled carbon nanotubes over ceria, zirconia and lanthana supported nickel catalysts prepared via a facile solid state citrate fusion method. Energy Convers. Manag. 2016, 126, 302–315. [Google Scholar] [CrossRef]
- Mendoza-Nieto, J.A.; Vera, E.; Pfeiffer, H. Methane Reforming Process by means of a Carbonated Na2ZrO3 Catalyst. Chem. Lett. 2016, 45, 685–687. [Google Scholar] [CrossRef]
- Lašič Jurković, D.; Puliyalil, H.; Pohar, A.; Likozar, B. Plasma-activated methane partial oxidation reaction to oxygenate platform chemicals over Fe, Mo, Pd and zeolite catalysts. Int. J. Energy Res. 2019, 43, 8085–8099. [Google Scholar] [CrossRef]
- Zhang, H.; Du, C.; Wu, A.; Bo, Z.; Yan, J.; Li, X. Rotating gliding arc assisted methane decomposition in nitrogen for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 12620–12635. [Google Scholar] [CrossRef]
- da Costa Labanca, A.R. Carbon black and hydrogen production process analysis. Int. J. Hydrogen Energy 2020, 45, 25698–25707. [Google Scholar] [CrossRef]
- Nozaki, T.; Okazaki, K. Non-thermal plasma catalysis of methane: Principles, energy efficiency, and applications. Catal. Today 2013, 211, 29–38. [Google Scholar] [CrossRef]
- Wang, B.; Cao, X.; Yang, K.; Xu, G. Conversion of methane through dielectric-barrier discharge plasma. Front. Chem. Eng. China 2008, 2, 373–378. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Taghvaei, H.; Jahanmiri, A.; Rahimpour, M.R.; Shirazi, M.M.; Hooshmand, N. Hydrogen production through plasma cracking of hydrocarbons: Effect of carrier gas and hydrocarbon type. Chem. Eng. J. 2013, 226, 384–392. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.-H.; Song, Y.-H.; Choi, W.C.; Park, Y.-K.; Kim, D.H. Synergistic effect of non-thermal plasma–catalysis hybrid system on methane complete oxidation over Pd-based catalysts. Chem. Eng. J. 2015, 259, 761–770. [Google Scholar] [CrossRef]
- Kim, S.-S.; Kim, J.; Lee, H.; Na, B.-K.; Song, H.K. Methane conversion over nanostructured Pt/γ-Al2O3 catalysts in dielectric-barrier discharge. Korean J. Chem. Eng. 2005, 22, 585–590. [Google Scholar] [CrossRef]
- Indarto, A. Hydrogen production from methane in a dielectric barrier discharge using oxide zinc and chromium as catalyst. J. Chin. Inst. Chem. Eng. 2008, 39, 23–28. [Google Scholar] [CrossRef]
- Son, I.H.; Kwon, S.; Park, J.H.; Lee, S.J. High coke-resistance MgAl2O4 islands decorated catalyst with minimizing sintering in carbon dioxide reforming of methane. Nano Energy 2016, 19, 58–67. [Google Scholar] [CrossRef]
- Li, G.; Cheng, H.; Zhao, H.; Lu, X.; Xu, Q.; Wu, C. Hydrogen production by CO2 reforming of CH4 in coke oven gas over Ni−Co/MgAl2O4 catalysts. Catal. Today 2018, 318, 46–51. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Saidina Amin, N.A. Process optimization of DBD plasma dry reforming of methane over Ni/La2O3-MgAl2O4 using multiple response surface methodology. Int. J. Hydrogen Energy 2019, 44, 11774–11787. [Google Scholar] [CrossRef]
- Guo, J.J.; Lou, H.; Zhao, H.; Chai, D.F.; Zheng, X.M. Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl Catal A-Gen 2004, 273, 75–82. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Saidina Amin, N.A. Evaluating the Performance of a Ni Catalyst Supported on La2O3-MgAl2O4 for Dry Reforming of Methane in a Packed Bed Dielectric Barrier Discharge Plasma Reactor. Energy Fuels 2019, 33, 11630–11647. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, H. Simultaneous production of hydrogen and carbon nanotubes from cracking of a waste cooking oil model compound over Ni-Co/SBA-15 catalysts. Int. J. Energy Res. 2020. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Avraam, D.G.; Goula, M.A. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Jamil, U.; Husain Khoja, A.; Liaquat, R.; Raza Naqvi, S.; Nor Nadyaini Wan Omar, W.; Aishah Saidina Amin, N. Copper and calcium-based metal organic framework (MOF) catalyst for biodiesel production from waste cooking oil: A process optimization study. Energy Convers. Manag. 2020, 215, 112934. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Dry reforming of methane using different dielectric materials and DBD plasma reactor configurations. Energy Convers. Manag. 2017, 144, 262–274. [Google Scholar] [CrossRef]
- Sanjabi, S.; Obeydavi, A. Synthesis and characterization of nanocrystalline MgAl2O4 spinel via modified sol–gel method. J. Alloy. Compd. 2015, 645, 535–540. [Google Scholar] [CrossRef]
- Nishikawa, H.; Kawamoto, D.; Yamamoto, Y.; Ishida, T.; Ohashi, H.; Akita, T.; Honma, T.; Oji, H.; Kobayashi, Y.; Hamasaki, A.; et al. Promotional effect of Au on reduction of Ni(II) to form Au-Ni alloy catalysts for hydrogenolysis of benzylic alcohols. J. Catal. 2013, 307, 254–264. [Google Scholar] [CrossRef]
- Wang, C.; Sun, N.; Zhao, N.; Wei, W.; Zhao, Y. Template-free preparation of bimetallic mesoporous Ni-Co-CaO-ZrO2 catalysts and their synergetic effect in dry reforming of methane. Catal. Today 2017, 281, 268–275. [Google Scholar] [CrossRef]
- Malekabadi, M.A.; Mamoory, R.S. Low-temperature synthesis of micro/nano Lithium Fluoride added magnesium aluminate spinel. Ceram. Int. 2018, 44, 20122–20131. [Google Scholar] [CrossRef]
- Ray, D.; Reddy, P.M.K.; Subrahmanyam, C. Ni-Mn/γ-Al2O3 assisted plasma dry reforming of methane. Catal. Today 2018, 309, 212–218. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, Y.; Jin, Y. Dry reforming of methane in an atmospheric pressure plasma fluidized bed with Ni/γ-Al2O3 catalyst. Catal. Today 2009, 148, 275–282. [Google Scholar] [CrossRef]
- Heintze, M.; Pietruszka, B. Plasma catalytic conversion of methane into syngas: The combined effect of discharge activation and catalysis. Catal. Today 2004, 89, 21–25. [Google Scholar] [CrossRef]
- Jiang, T.; Li, Y.; Liu, C.J.; Xu, G.H.; Eliasson, B.; Xue, B.Z. Plasma methane conversion using dielectric-barrier discharges with zeolite A. Catal. Today 2002, 72, 229–235. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Cold plasma dielectric barrier discharge reactor for dry reforming of methane over Ni/ɤ-Al2O3 -MgO nanocomposite. Fuel Process. Technol. 2018, 178, 166–179. [Google Scholar] [CrossRef]
- Neyts, E.C.; Ostrikov, K.K.; Sunkara, M.K.; Bogaerts, A. Plasma catalysis: Synergistic effects at the nanoscale. Chem. Rev. 2015, 115, 13408–13446. [Google Scholar] [CrossRef] [PubMed]
- Neyts, E.C.; Ostrikov, K. Nanoscale thermodynamic aspects of plasma catalysis. Catal. Today 2015, 256, 23–28. [Google Scholar] [CrossRef]
- Yap, D.; Tatibouët, J.-M.; Batiot-Dupeyrat, C. Catalyst assisted by non-thermal plasma in dry reforming of methane at low temperature. Catal. Today 2018, 299, 263–271. [Google Scholar] [CrossRef]
- Liu, C.J.; Li, M.Y.; Wang, J.Q.; Zhou, X.T.; Guo, Q.T.; Yan, J.M.; Li, Y.Z. Plasma methods for preparing green catalysts: Current status and perspective. Chin. J. Catal. 2016, 37, 340–348. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, K.; Rui, N.; Zhao, B.; Liu, C.-j. Improved activity of Ni/MgAl2O4 for CO2 methanation by the plasma decomposition. J. Energy Chem. 2015, 24, 655–659. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zhao, H.; Zheng, X. Improvement of stability of out-layer MgAl2O4 spinel for a Ni/MgAl2O4/Al2O3 catalyst in dry reforming of methane. React. Kinet. Catal. Lett. 2005, 84, 93–100. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zheng, X. The deposition of coke from methane on a Ni/MgAl2O4 catalyst. Carbon 2007, 45, 1314–1321. [Google Scholar] [CrossRef]
- Megía, P.J.; Calles, J.A.; Carrero, A.; Vizcaíno, A.J. Effect of the incorporation of reducibility promoters (Cu, Ce, Ag) in Co/CaSBA-15 catalysts for acetic acid steam reforming. Int. J. Energy Res. 2020. [Google Scholar] [CrossRef]
- Osman, A.I.; Blewitt, J.; Abu-Dahrieh, J.K.; Farrell, C.; Al-Muhtaseb, A.H.; Harrison, J.; Rooney, D.W. Production and characterisation of activated carbon and carbon nanotubes from potato peel waste and their application in heavy metal removal. Env. Sci. Pollut. Res. Int. 2019, 26, 37228–37241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnia, L.; Kumari, R.; Kumar, V.; Singh, A.; Garg, P.; Yadav, B.S.; Tyagi, P.K. Comparative study of thermal stability of filled and un-filled multiwalled carbon nanotubes. Adv. Mater. Lett. 2016, 7, 230–234. [Google Scholar] [CrossRef]
- Azmina, M.; Suriani, A.B.; Salina, M.; Azira, A.; Dalila, A.; Asli, N.; Rosly, J.; Nor, R.M.; Rusop, M. Variety of Bio-Hydrocarbon Precursors for the Synthesis of Carbon Nanotubes; Trans Tech Publisher: Baech, Switzerland, 2012; pp. 43–63. [Google Scholar]
- Pudukudy, M.; Yaakob, Z.; Takriff, M.S. Methane decomposition over Pd promoted Ni/MgAl2O4 catalysts for the production of COx free hydrogen and multiwalled carbon nanotubes. Appl. Surf. Sci. 2015, 356, 1320–1326. [Google Scholar] [CrossRef]
- Dong, Z.; Li, B.; Cui, C.; Qian, W.; Jin, Y.; Wei, F. Catalytic methane technology for carbon nanotubes and graphene. React. Chem. Eng. 2020, 5, 991–1004. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoja, A.H.; Azad, A.K.; Saleem, F.; Khan, B.A.; Naqvi, S.R.; Mehran, M.T.; Amin, N.A.S. Hydrogen Production from Methane Cracking in Dielectric Barrier Discharge Catalytic Plasma Reactor Using a Nanocatalyst. Energies 2020, 13, 5921. https://0-doi-org.brum.beds.ac.uk/10.3390/en13225921
Khoja AH, Azad AK, Saleem F, Khan BA, Naqvi SR, Mehran MT, Amin NAS. Hydrogen Production from Methane Cracking in Dielectric Barrier Discharge Catalytic Plasma Reactor Using a Nanocatalyst. Energies. 2020; 13(22):5921. https://0-doi-org.brum.beds.ac.uk/10.3390/en13225921
Chicago/Turabian StyleKhoja, Asif Hussain, Abul Kalam Azad, Faisal Saleem, Bilal Alam Khan, Salman Raza Naqvi, Muhammad Taqi Mehran, and Nor Aishah Saidina Amin. 2020. "Hydrogen Production from Methane Cracking in Dielectric Barrier Discharge Catalytic Plasma Reactor Using a Nanocatalyst" Energies 13, no. 22: 5921. https://0-doi-org.brum.beds.ac.uk/10.3390/en13225921