Production of Fuel-Like Fractions by Fractional Distillation of Bio-Oil from Açaí (Euterpe oleracea Mart.) Seeds Pyrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methodology
2.2. Materials
2.3. Pre-Treatment of Açaí Seeds in Nature
2.4. Centesimal Characterization of Açaí Seeds
2.5. Experimental Apparatus and Procedures
2.5.1. Pyrolysis Units
2.5.2. Experimental Procedures
2.5.3. Distillation Unit
2.6. Physical-Chemistry Analysis and Chemical Composition of Bio-Oils and Distillation Fractions
2.6.1. Physical-Chemistry Analysis of Distillation Fractions
2.6.2. Chemical Composition of Bio-Oils and Distillation Fractions
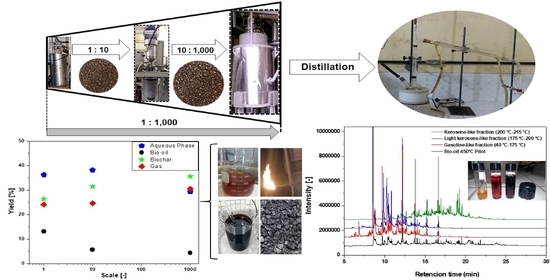
3. Results
3.1. Pre-Treatments and Centesimal Characterization of Açaí Seeds
3.2. Pyrolysis of Açaí Seeds
3.2.1. Material Balances, Operating Conditions, and Yields of Reaction Products
3.2.2. Physical-Chemistry Characterization of Bio-Oil
3.3. Distillation of Bio-Oil from Pyrolysis of Açaí Seeds
Physico-Chemical Characterization of Distillation Fractions
3.4. FT-IR and GC-MS Analyses of Bio-Oil and Distillation Fractions
3.4.1. FT-IR Spectroscopy of Bio-Oil and Distillation Fractions
3.4.2. Chemical Compositional of Bio-Oil and Distillation Fractions by GC-MS
Chemical Compositional of Bio-Oils by GC-MS
Chemical Compositional of Distillation Fractions by GC-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scherwinski-Pereira, J.E.; Guedes, R.D.S.; Da Silva, R.A.; Fermino, P.C.P.; Luis, Z.G.; Freitas, E.D.O. Somatic embryogenesis and plant regeneration in açaí palm (Euterpe oleracea). Plant Cell Tissue Organ Cult. (PCTOC) 2012, 109, 501–508. [Google Scholar] [CrossRef]
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Patel, D.; Huang, D.; Kababick, J.P. Phytochemical and Nutrient Composition of the Freeze-Dried Amazonian Palm Berry, Euterpe oleraceaeMart. (Acai). J. Agric. Food Chem. 2006, 54, 8598–8603. [Google Scholar] [CrossRef] [PubMed]
- Sabbe, S.; Verbeke, W.; Deliza, R.; Matta, V.; Van Damme, P. Effect of a health claim and personal characteristics on consumer acceptance of fruit juices with different concentrations of açaí (Euterpe oleracea Mart.). Appetite 2009, 53, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Palencia, L.A.; Duncan, C.E.; Talcott, S.T. Phytochemical composition and thermal stability of two commercial açai species, Euterpe oleracea and Euterpe precatoria. Food Chem. 2009, 115, 1199–1205. [Google Scholar] [CrossRef]
- Brondizio, E.S.; Safar, C.A.; Siqueira, A.D. The urban market of Açaí fruit (Euterpe oleracea Mart.) and rural land use change: Ethnographic insights into the role of price and land tenure constraining agricultural choices in the Amazon estuary. Urban Ecosyst. 2002, 6, 67–97. [Google Scholar] [CrossRef]
- Dos Santos Bentes, E.; Oyama Homma, A.K.; Nunes dos Santos, C.A. Exportações de Polpa de Açaí do Estado do Pará: Situação Atual e Perspectivas. In: Anais Congresso da Sociedade Brasileira de Economia, Administração e Sociologia Rural, 55, Santa Maria, RS-Brazil. 2017. Available online: https://www.researchgate.net/publication/319465735_Exportacoes_de_Polpa_de_Acai_do_Estado_do_Para_Situacao_Atual_e_Perspectivas (accessed on 5 August 2020).
- Almeida, A.V.D.C.; Melo, I.M.; Pinheiro, I.S.; Freitas, J.F. Appreciation of acai core of a pulp producer from Ananindeua/PA: Proposal of reverse channel structure oriented by NPSW and reverse logistics. Rev. Gestão Produção Operações Sist. 2017, 12, 59–83. [Google Scholar] [CrossRef]
- Townsend, C.R.; de Lucena Costa, N.; de Araújo Pereira, R.G.; Clóvis, C. Diesel Senger. Características Químico-Bromatológica do Caroço de Açaí. COMUNICADO TÉCNICO Nº 193 (CT/193), EMBRAPA-CPAF Rondônia, ago./01, 1-5. ISSN 0103-9458. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/100242/1/Cot193-acai.pdf (accessed on 5 August 2020).
- Fioravanti, C. Açaí: Do pé para o lanche. Rev. Pesqui. Fapesp. 2013, 203, 64–68. Available online: http://revistapesquisa.fapesp.br/2013/01/11/folheie-a-edicao-203/ (accessed on 5 August 2020).
- De Santana, A.C.; De Santana, Á.L.; De Santana, Á.L.; Dos Santos, M.A.S.; De Oliveira, C.M. Análise discriminante múltipla do mercado varejista de açaí em Belém do Pará. Rev. Bras. Frutic. 2014, 36, 532–541. [Google Scholar] [CrossRef] [Green Version]
- Pessoa, J.D.C.; Silva, P.V.D. Effect of temperature and storage on açaí (Euterpe oleracea) fruit water uptake: Simulation of fruit transportation and pre-processing. Fruits 2007, 62, 295–302. [Google Scholar] [CrossRef]
- Cordeiro, M.A. Estudo da Hidrólise Enzimática do Caroço de açaí (Euterpe Oleracea, Mart) para a Produção de Etanol. Dissertação de Mestrado, Programa de Pós-Graduação em Engenharia Química, UFPA-Brazil. Marcio de Andrade Cordeiro. 2016. Available online: https://ppgeq.propesp.ufpa.br/ARQUIVOS/dissertacoes/2016/M%C3%A1rcio%20de%20Andrade%20Cordeiro/M%C3%A1rcio_Disserta%C3%A7%C3%A3o_Defesa.pdf (accessed on 5 August 2020).
- Da Fonseca, T.R.; de Amorim Silva, T.; Alecrim, M.M.; da Cruz Filho, R.F.; Teixeira, M.F. Cultivation and nutritional studies of an edible mushroom from North Brazil. Afr. J. Microbiol. Res. 2015, 9, 1814–1822. [Google Scholar]
- Kabacknik, A.; Roger, H. Determinação do Poder Calorífico do Caroço do açaí em três Distintas Umidades. In Proceedings of the 38th Congresso Brasileiro de Química, São Luiz, MA, Brazil, 21–25 September 1998. [Google Scholar]
- Altman, R.F.A. O Caroço de açaí (Euterpe Oleracea, Mart); Boletim Técnico do Instituto Agronômico do Norte: Belém, Brasil, 1956; Volume 31, pp. 109–111. Available online: https://www.bdpa.cnptia.embrapa.br/consulta/busca?b=ad&biblioteca=vazio&busca=autoria:"ALTMAN,%20R.%20F.%20A." (accessed on 5 August 2020).
- Özçimen, D.; Ersoy-Meriçboyu, A. Characterization of biochar and bio-oil samples obtained from carbonization of various biomass materials. Renew. Energy 2010, 35, 1319–1324. [Google Scholar] [CrossRef]
- Adjaye, J.D.; Sharma, R.K.; Bakhshi, N.N. Characterization and stability analysis of wood-derived bio-oil. Fuel Process. Technol. 1992, 31, 241–256. [Google Scholar] [CrossRef]
- Carazza, F.; Rezende, M.E.A.; Pasa, V.M.D.; Lessa, A. Fractionation of wood tar. Proc. Adv. Thermochem. Biomass Convers. 1994, 2, 465. [Google Scholar]
- Adjaye, J.; Bakhshi, N. Production of hydrocarbons by catalytic upgrading of a fast pyrolysis bio-oil. Part I: Conversion over various catalysts. Fuel Process. Technol. 1995, 45, 161–183. [Google Scholar] [CrossRef]
- Xu, B.J.; Lu, N. Experimental research on the bio-oil derived from biomass pyrolysis liquefaction. Trans. Chin. Soc. Agric. Eng. 1999, 15, 177–181. [Google Scholar]
- Boucher, M.E.; Chaala, A.; Roy, C. Bio-oils obtained by vacuum pyrolysis of softwood bark as a liquid fuel for gas turbines. Part I: Properties of bio-oil and its blends with methanol and a pyrolytic aqueous phase. Biomass Bioenergy 2000, 19, 337–350. [Google Scholar] [CrossRef]
- Oasmaa, A.; Kuoppala, E.; Gust, S.; Solantausta, Y. Fast Pyrolysis of Forestry Residue. 1. Effect of Extractives on Phase Separation of Pyrolysis Liquids. Energy Fuels 2003, 17, 1–12. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Steelee, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Yu, F.; Deng, S.; Chen, P.; Liu, Y.; Wan, Y.; Olson, A.; Kittelson, D.; Ruan, R. Physical and Chemical Properties of Bio-Oils from Microwave Pyrolysis of Corn Stover. Appl. Biochem. Biotecnol. 2007, 136–140, 957–970. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, J.; Wang, T.; Xu, Y. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers. Manag. 2007, 48, 87–92. [Google Scholar] [CrossRef]
- Boateng, A.A.; Mullen, C.A.; Goldberg, N.; Hicks, K.B. Production of bio-oil from alfalfa stems by fluidized-bed fast pyrolysis. Ind. Eng. Chem. Res. 2008, 47, 4115–4122. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, X.L.; Zhu, X.F. Analysis on chemical and physical properties of bio-oil pyrolyzed from rice husk. J. Anal. Appl. Pyrolysis 2008, 82, 191–198. [Google Scholar] [CrossRef]
- Junming, X.; Jianchun, J.; Yunjuan, S.; Yanju, L. Bio-oil upgrading by means of ethyl ester production in reactive distillation to remove water and to improve storage and fuel characteristics. Biomass Bioenergy 2008, 32, 1056–1061. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, S.; Zhu, Y.; Luo, Z.; Cen, K. Separation of acid compounds for refining biomass pyrolysis oil. J. Fuel Chem. Technol. 2009, 7, 49–52. [Google Scholar] [CrossRef]
- Vispute, T.P.; Huber, G.W. Production of hydrogen, alkanes and polyols by aqueous phase processing of wood-derived pyrolysis oils. Green Chem. 2009, 11, 1433–1445. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.-H.; Nie, J.-Q.; Ren, M.-G.; Guo, Q.-X. Effective Phase Separation of Biomass Pyrolysis Oils by Adding Aqueous Salt Solutions. Energy Fuels 2009, 23, 3307–3312. [Google Scholar] [CrossRef]
- Wang, S.; Gu, Y.; Liu, Q.; Yao, Y.; Guo, Z.; Luo, Z.; Cen, K. Separation of bio-oil by molecular distillation. Fuel Process. Technol. 2009, 90, 738–745. [Google Scholar] [CrossRef]
- Oasmaa, A.; Elliott, D.C.; Korhonen, J. Acidity of Biomass Fast Pyrolysis Bio-oils. Energy Fuels 2010, 24, 6548–6554. [Google Scholar] [CrossRef]
- De Castro, D.A.R. Processo de Produção de Bio-Óleo e Bio-Adsorventes via Pirólise das Sementes do Açaí (Euterpe oleraceae, Mart). Ph.D. Thesis, PRODERNA, UFPa, Belém, Brazil, 2019. [Google Scholar]
- Guo, X.; Wang, S.; Guo, Z.; Liu, Q.; Luo, Z.; Cen, K. Pyrolysis characteristics of bio-oil fractions separated by molecular distillation. Appl. Energy 2010, 87, 2892–2898. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, S.; Gu, Y.; Xu, G.; Li, X.; Luo, Z. Separation characteristics of biomass pyrolysis oil in molecular distillation. Sep. Purif. 2010, 76, 52–57. [Google Scholar] [CrossRef]
- Suota, M.J.; Simionatto, E.L.; Scharf, D.R.; Meier, H.F.; Wiggers, V.R. Esterification, Distillation, and Chemical Characterization of Bio-Oil and Its Fractions. Energy Fuels 2019, 33, 9886–9894. [Google Scholar] [CrossRef]
- Nam, H.; Choi, J.; Capareda, S.C. Comparative study of vacuum and fractional distillation using pyrolytic microalgae (Nannochloropsis oculata) bio-oil. Algal Res. 2016, 17, 87–96. [Google Scholar] [CrossRef]
- Christensen, E.D.; Chupka, G.M.; Smurthwaite, J.L.T.; Alleman, T.L.; Lisa, K.; Franz, J.A.; Elliott, D.C.; Mc Cormick, R.L. Analysis of oxygenated compounds in hydrotreated biomass fast pyrolysis oil distillate fractions. Energy Fuels 2011, 25, 5462–5471. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Wei, Q. Improving the quality of fast pyrolysis bio-oil by reduced pressure distillation. Biomass Bioenergy 2011, 35, 1804–1810. [Google Scholar] [CrossRef]
- Pollard, A.; Rover, M.; Brown, R.C. Characterization of bio-oil recovered as stage fractions with unique chemical and physical properties. J. Anal. Appl. Pyrolysis 2012, 93, 129–138. [Google Scholar] [CrossRef]
- Shah, A.; Darr, M.J.; Dalluge, D.; Medic, D.; Webster, K.; Brown, R.C. Physicochemical properties of bio-oil and biochar produced by fast pyrolysis of stored single-pass corn stover and cobs. Bioresour. Technol. 2012, 125, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Imam, T.; Capareda, S. Characterization of bio-oil, syngas and bio-char from switch grass pyrolysis at various temperatures. J. Anal. Appl. Pyrolysis 2012, 93, 170–177. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A. Bio-oil production and upgrading research: A review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- Majhi, A.; Sharma, Y.K.; Naik, D.V. Blending optimization of Hempel distilled bio-oil with commercial diesel. Fuel 2012, 96, 264–269. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Yang, G.-X.; Jiang, H.; Liu, W.-J.; Ding, H.-S. Mass production of chemicals from biomass-derived oil by directly atmospheric distillation coupled with co-pyrolysis. Sci. Rep. 2013, 3, srep01120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capunitan, J.A.; Capareda, S.C. Characterization and separation of corn stover bio-oil by fractional distillation. Fuel 2013, 112, 60–73. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Yang, H.; Yao, J.; Chen, G.; Ma, W.; Yan, B.; Qi, Y. Overview of Upgrading of Pyrolysis Oil of Biomass. Energy Procedia 2014, 61, 1306–1309. [Google Scholar] [CrossRef] [Green Version]
- Gooty, A.T.; Li, D.; Berruti, F.; Briens, C. Kraft-lignin pyrolysis and fractional condensation of its bio-oil vapors. J. Anal. Appl. Pyrolysis 2014, 106, 33–40. [Google Scholar] [CrossRef]
- Biradar, C.H.; Subramanian, K.A.; Dastidar, M.G. Production and fuel upgrading of pyrolysis bio-oil Jatropha Curcas de-oiled seed cake. Fuel 2014, 119, 81–89. [Google Scholar] [CrossRef]
- Elkasabi, Y.; Mullen, C.A.; Boateng, A.A. Distillation and isolation of commodity chemicals from bio-oil made by tail-gas reactive pyrolysis. Sustain. Chem. Eng. 2014, 2, 2042–2052. [Google Scholar] [CrossRef]
- Gooty, A.T.; Li, D.; Briens, C.; Berruti, F. Fractional condensation of bio-oil vapors produced from birch bark pyrolysis. Sep. Purif. Technol. 2014, 124, 81–88. [Google Scholar] [CrossRef]
- Wang, S.; Cai, Q.; Wang, X.; Zhang, L.; Wang, Y.; Luo, Z. Biogasoline Production from the Co-cracking of the Distilled Fraction of Bio-oil and Ethanol. Energy Fuels 2013, 28, 115–122. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K. A review on the pyrolysis of woody biomass to bio-oil: Focus on kinetic models. Renew. Sustain. Energy Rev. 2015, 52, 1580–1595. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydro-char in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Kumar, S.; Lange, J.-P.; Van Rossum, G.; Kersten, S.R. Bio-oil fractionation by temperature-swing extraction: Principle and application. Biomass Bioenergy 2015, 83, 96–104. [Google Scholar] [CrossRef]
- Elkasabi, Y.; Boateng, A.A.; Jackson, M.A. Upgrading of bio-oil distillation bottoms into biorenewable calcined coke. Biomass Bioenergy 2015, 81, 415–423. [Google Scholar] [CrossRef]
- Elkasabi, Y.; Mullen, C.A.; Jackson, M.A.; Boateng, A.A. Characterization of fast-pyrolysis bio-oil distillation residues and their potential applications. J. Anal. Appl. Pyrolysis 2015, 114, 179–186. [Google Scholar] [CrossRef]
- Kanaujia, P.K.; Naik, D.V.; Tripathi, D.; Singh, R.; Poddar, M.K.; Siva Kumar Konathala, L.N.; Sharma, Y.K. Pyrolysis of Jatropha Curcas seed cake followed by optimization of liquid–liquid extraction procedure for the obtained bio-oil. Anal. Appl. Pyrolysis 2016, 118, 202–224. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Cai, J.; Banks, S.W.; Yang, Y.; Darbar, S.; Bridgwater, T. Viscosity of Aged Bio-oils from Fast Pyrolysis of Beech Wood and Miscanthus: Shear Rate and Temperature Dependence. Energy Fuels 2016, 30, 4999–5004. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Wang, F.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z.; Gu, J. Study on aromatics production via the catalytic pyrolysis vapor upgrading of biomass using metal-loaded modified H-ZSM-5. J. Anal. Appl. Pyrolysis 2017, 126, 169–179. [Google Scholar] [CrossRef]
- Johansson, A.-C.; Iisa, K.; Sandström, L.; Ben, H.; Pilath, H.; Deutch, S.; Wiinikka, H.; Öhrman, O.G. Fractional condensation of pyrolysis vapors produced from Nordic feedstocks in cyclone pyrolysis. J. Anal. Appl. Pyrolysis 2017, 123, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.P.; Hou, B.R.; Huang, A.N. The influence of the gas fluidization velocity on the properties of bio-oils from fluidized bed pyrolizer with in-line distillation. Appl. Energy 2017, 194, 279–286. [Google Scholar] [CrossRef]
- Guedes, R.E.; Luna, A.S.; Torres, A.R. Operating parameters for bio-oil production in biomass pyrolysis: A review. J. Anal. Appl. Pyrolysis 2018, 129, 134–149. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Cai, W.; Liu, R.; He, Y.; Chai, M.; Cai, J. Bio-oil production from fast pyrolysis of rice husk in a commercial-scale plant with a downdraft circulating fluidized bed reactor. Fuel Process. Technol. 2018, 171, 308–317. [Google Scholar] [CrossRef]
- Huang, A.-N.; Hsu, C.-P.; Hou, B.-R.; Kuo, H.-P. Production and separation of rice husk pyrolysis bio-oils from a fractional distillation column connected fluidized bed reactor. Powder Technol. 2018, 323, 588–593. [Google Scholar] [CrossRef]
- Rahman, S.; Helleur, R.; MacQuarrie, S.; Papari, S.; Hawboldt, K. Upgrading and isolation of low molecular weight compounds from bark and softwood bio-oils through vacuum distillation. Sep. Purif. Technol. 2018, 194, 123–129. [Google Scholar] [CrossRef]
- Yuan, X.; Sun, M.; Wang, C.; Zhu, X. Full temperature range study of rice husk bio-oil distillation: Distillation characteristics and product distribution. Sep. Purif. Technol. 2021, 263, 118382. [Google Scholar] [CrossRef]
- De Castro, D.A.R.; Ribeiro, H.J.D.S.; Ferreira, C.C.; Cordeiro, M.D.A.; Guerreiro, L.H.H.; Pereira, A.M.; Dos Santos, W.G.; Santos, M.C.; De Carvalho, F.B.; Junior, J.O.C.S.; et al. Fractional Distillation of Bio-Oil Produced by Pyrolysis of Açaí (Euterpe oleracea) Seeds. In Fractionation; Ibrahim, H.A.-H., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- De Andrade Cordeiro, M.; de Almeida, O.; de Castro, D.A.; da Silva Ribeiro, H.J.; Machado, N.T. Produção de Etanol através da Hidrólise Enzimática do Caroço de Açaí (Euterpe oleracea, Mart.). Rev. Bras. Energ. Renov. 2019, 8, 122–152. [Google Scholar]
- Acid-Insoluble Lignin in Wood and Pulp; Tappi Method T 222 Om-06; Tappi Press: Atlanta, GA, USA, 2006.
- Buffiere, P.; Loisel, D. Dosage des fibres Van Soest; Weened, Laboratoire de Biotechnologie de l’Environnement, INRA: Narbonne, France, 2007; pp. 1–14. [Google Scholar]
- Almeida, H.D.S.; Corrêa, O.; Eid, J.; Ribeiro, H.; De Castro, D.; Pereira, M.; Pereira, L.; Aâncio, A.D.A.; Santos, M.; Da Mota, S.; et al. Performance of thermochemical conversion of fat, oils, and grease into kerosene-like hydrocarbons in different production scales. J. Anal. Appl. Pyrolysis 2016, 120, 126–143. [Google Scholar] [CrossRef]
- Da Mota, S.A.; Mancio, A.A.; Lhamas, D.E.; De Abreu, D.H.; Da Silva, M.S.; Dos Santos, W.G.; De Castro, D.A.; De Oliveira, R.M.; Araújo, M.E.; Borges, L.E.; et al. Production of green diesel by thermal catalytic cracking of crude palm oil (Elaeis guineensis Jacq) in a pilot plant. J. Anal. Appl. Pyrolysis 2014, 110, 1–11. [Google Scholar] [CrossRef]
- Ferreira, C.C.; Costa, E.C.; de Castro, D.A.; Pereira, M.S.; Mâncio, A.A.; Santos, M.C.; Lhamas, D.E.; Da Mota, S.A.; Leão, A.C.; Duvoisin, S., Jr.; et al. Deacidification of organic liquid products by fractional distillation in laboratory and pilot scales. J. Anal. Appl. Pyrolysis 2017, 127, 468–489. [Google Scholar] [CrossRef]
- Seshadri, K.S.; Cronauer, D.C. Characterization of coal-derived liquids by 13C N.M.R. and FT-IR Spectroscopy. Fuel 1983, 62, 1436–1444. [Google Scholar] [CrossRef]
- Abnisa, F.; Arami-Niya, A.; Daud, W.M.; Sahu, J.N. Characterization of Bio-oil and Bio-char from Pyrolysis of Palm Oil Wastes. BioEnergy Res. 2013, 6, 830–840. [Google Scholar] [CrossRef]
- Tanneru, S.K.; Parapati, D.R.; Steele, P.H. Pretreatment of bio-oil followed by upgrading via esterification to boiler fuel. Energy 2014, 73, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Perez, M.; Chaala, A.; Roy, C. Vacuum pyrolysis of sugarcane bagasse. J. Anal. Appl. Pyrolysis 2002, 65, 111–136. [Google Scholar] [CrossRef]
- Nolte, M.W.; Liberatore, M.W. Viscosity of Biomass Pyrolysis Oils from Various Feedstocks. Energy Fuels 2010, 24, 6601–6608. [Google Scholar] [CrossRef]
- Ba, T.; Chaala, A.; Garcia-Perez, M.; Rodrigue, D.; Roy, C. Colloidal properties of bio-oils obtained by vacuum pyrolysis of softwood bark. Characterization of water-soluble and water-insoluble fractions. Energy Fuels 2004, 18, 704–712. [Google Scholar] [CrossRef]













| Injetor | Injection | T (°C) | Flow Rate (mL/min) | Split |
|---|---|---|---|---|
| 6.0 | 1:50 | |||
| 250 | Heating Rate (°C/min) | Volume (μL) | ||
| 10 | 1.0 | |||
| Oven | Heating Rate (°C/min) | T (°C) | Residence Time (min) | |
| - | 60 | 1 | ||
| 5 | 200 | 2 | ||
| 20 | 230 | 10 | ||
| 10 | 280 | 39 | ||
| Detector (MS) | T (°C) | Carrier Gas | Flow Rate (mL/min) | T (°C) Quadrupole |
| 230 | He | 30.0 | 150 | |
| Temperature | |||
|---|---|---|---|
| Process Parameters | 450 (°C) | 450 (°C) | 450 (°C) |
| Pilot | Bench | Laboratory | |
| Mass of Açaí Seeds (kg) | 30 | 900 × 10−3 | 50.49 × 10−3 |
| Mass of LPG (g) | 13.33 | - | - |
| Cracking Time (min) | 160 | 90 | 53 |
| Initial Cracking Temperature (°C) | 117 | 115 | 310 |
| Mass of Liquid (Bio-Oil + H2O) (kg) | 11.40 | 394.07 × 10−3 | 24.95 × 10−3 |
| Mass of Coke (kg) | 10.50 | 283.97 × 10−3 | 13.35 × 10−3 |
| Mass of Bio-Oil (kg) | 1.31 | 50.40 × 10−3 | 6.61 × 10−3 |
| Mass of H2O (kg) | 10.09 | 343.67 × 10−3 | 18.34 × 10−3 |
| Mass of Gas (kg) | 8.10 | 221.96 × 10−3 | 12.19 × 10−3 |
| Yield of Bio-Oil (wt.%) | 4.37 | 6.60 | 13.09 |
| Yield of Coke (wt.%) | 35.00 | 31.55 | 26.44 |
| Yield of H2O (wt.%) | 33.63 | 38.19 | 36.32 |
| Yield of Gas (wt.%) | 27.00 | 24.66 | 24.14 |
| Physicochemical Properties | 450 °C Pilot | 450 °C Laboratory | 450 °C Bench | [21] | [25] | [28] | [41] | [69] | [82] | [81] | ANP No 65 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bio-Oil | Bio-Oil | Bio-Oil | Bio-Oil | Bio-Oil | Bio-Oil | Bio-Oil | Bio-Oil | Bio-Oil | Bio-Oil | ||
| ρ (g/cm3), 30 °C | 1.043 | ND | ND | 1.066 | 1.250 | 1.140 | 1.190 | 1.1581 | 1.200 | 1.030 | 0.82–0.85 |
| I. A (mg KOH/g) | 70.26 | 70.25 ± 1.00 | 68.31 ± 0.90 | - | - | - | - | - | - | - | |
| ν (mm2/s), 40 °C | 68.34 | ND | ND | 38.0 | 148.0 | 13.2 | 40.0 * | 5.0–13.0 | 12.0 | - | 2.0–4.5 |
| Physicochemical Properties | 450 °C Pilot | 450 °C Laboratory | 450 °C Bench | [43] | [48] | [83] | [84] | [84] | [84] | [84] | [84] | [84] | [84] | [84] | [85] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bio-Oil | Bio-Oil | Bio-Oil | Bio-Oil | Bio-Oil | Bio-Oil | Douglas Fir | Hardwood | Oak | Poplar | Pine | Softwood | Switch-Grass | Wheat Straw | Bio-Oil | |
| I. A [mg KOH/g] | 70.26 | 70.25 ± 1.00 | 68.31 ± 0.90 | 95.00 | 24.00 | 82.00 | 124.00 | 91.70 | 133.00 | 129.00 | 91.60 | 115.00 | 125.00 | 94.90 | 47.70 |
| Distillation: Vigreux Column | Bio-Oil (g) | Gas | Raffinate | Distillates (g) | Yield (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g) | (g) | (g) | H2O | G | LK | K | LD | H2O | G | LK | K | LD | |
| (30–215 °C) | 307.53 | 0 | 69.87 | 0 | 49.48 | 59.91 | 128.27 | 0 | 0 | 16.16 | 19.56 | 41.89 | 0 |
| Physico-Chemical Properties | 450 °C | ANP N° 65 | ||
|---|---|---|---|---|
| G | LK | K | ||
| ρ (g/cm3), 30 °C | 0.9146 | 0.9191 | 0.9816 | 0.82–0.85 |
| I. A (mg KOH/g) | 14.94 | 61.08 | 64.78 | |
| I. R (-) | 1.455 | 1.479 | 1.497 | |
| ν (mm2/s), 40 °C | 1.457 | 3.106 | 4.040 | 2.0–4.5 |
| Wave-Length (cm−1) | Functional Groups | Bio-Oil | ||
|---|---|---|---|---|
| 450 °C Pilot | 450 °C Bench | 450 °C Laboratory | ||
| 3380 | O–H hydroxyl (polymeric association) | X | X | X |
| 2960–2855 | C-H aliphatics (alkanes) | X | X | X |
| 1710 | C=O carbonyl (carboxylic acids) | X | X | X |
| 1595–1510 | C=C (Aromatic) | X | X | X |
| 1455–1465 | -CH2- angular deformation (methylene groups) | X | X | X |
| 1370 | CH3 angular deformation (dimethyl groups) | X | X | X |
| 1275–1020 | C-O (esters, ethers, alcohols and phenols) | X | X | X |
| 1225–1220 | C-O (phenols) | X | X | X |
| 1150 | C-O (tertiary alcohol) | - | X | - |
| 1110 | C-O (secondary alcohol) | X | - | X |
| 815–690 | C=C (adjacent 3H aromatic rings) | X | X | X |
| Classes of Chemical Compounds | ωi (Area.%) |
|---|---|
| Alkanes | |
| Σ (Area.%) | 7.521 |
| Alkenes | |
| Σ (Area.%) | 2.118 |
| Cycloalkenes | |
| Σ (Area.%) | 1.847 |
| Aromatics | |
| Σ (Area.%) | 10.038 |
| Esters | |
| Σ (Area.%) | 4.065 |
| Carboxylic Acids | |
| Σ (Area.%) | 8.523 |
| Ketones | |
| Σ (Area.%) | 3.533 |
| Phenols | |
| Σ (Area.%) | 35.167 |
| Cresols | |
| Σ (Area.%) | 20.526 |
| Furans | |
| Σ (Area.%) | 5.751 |
| Aldehydes | |
| Σ (Area.%) | 0.910 |
| Classes of Chemical Compounds | ωi (Area.%) |
|---|---|
| Alkanes | |
| Σ (Area.%) | 13.14 |
| Alkenes | |
| Σ (Area.%) | 25.50 |
| Aromatics | |
| Σ (Area.%) | 9.59 |
| Alcohols | |
| Σ (Area.%) | 0.43 |
| Ketones | |
| Σ (Area.%) | 4.43 |
| Phenols | |
| Σ (Area.%) | 30.87 |
| Furans | |
| Σ (Area.%) | 15.24 |
| Aldehydes | |
| Σ (Area.%) | 0.80 |
| Classes of Chemical Compounds | ωi (Area.%) |
|---|---|
| Alkanes | |
| Σ (Area.%) | 9.41 |
| Alkenes | |
| Σ (Area.%) | 13.27 |
| Aromatics | |
| Σ (Area.%) | 41.32 |
| Alcohols | |
| Σ (Area.%) | 6.05 |
| Esters | |
| Σ (Area.%) | 5.50 |
| Ketones | |
| Σ (Area.%) | 2.61 |
| Phenols | |
| Σ (Area.%) | 1.35 |
| Furans | |
| Σ (Area.%) | 13.24 |
| Aldehydes | |
| Σ (Area.%) | 7.25 |
| Classes of Chemical Compounds | ωi (Area.%) |
|---|---|
| Alkanes | |
| Σ (Area.%) | 32.65 |
| Alkenes | |
| Σ (Area.%) | 17.60 |
| Aromatics | |
| Σ (Area.%) | 16.42 |
| Esters | |
| Σ (Area.%) | 6.16 |
| Carboxylic Acids | |
| Σ (Area.%) | 3.26 |
| Ketones | |
| Σ (Area.%) | 4.24 |
| Phenols | |
| Σ (Area.%) | 7.13 |
| Alcohols | |
| Σ (Area.%) | 8.30 |
| Furans | |
| Σ (Area.%) | 2.39 |
| Aldehydes | |
| Σ (Area.%) | 1.86 |
| Classes of Chemical Compounds | ωi (Area.%) |
|---|---|
| Alkanes | |
| Σ (Area.%) | 4.20 |
| Alkenes | |
| Σ (Area.%) | 2.79 |
| Aromatics | |
| Σ (Area.%) | 12.88 |
| Alcohols | |
| Σ (Area.%) | 0.96 |
| Ethers | |
| Σ (Area.%) | 0.80 |
| Ketones | |
| Σ (Area.%) | 3.50 |
| Phenols | |
| Σ (Area.%) | 60.79 |
| Esters | |
| Σ (Area.%) | 2.06 |
| Furans | |
| Σ (Area.%) | 8.99 |
| Aldehydes | |
| Σ (Area.%) | 3.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha de Castro, D.A.; da Silva Ribeiro, H.J.; Hamoy Guerreiro, L.H.; Pinto Bernar, L.; Jonatan Bremer, S.; Costa Santo, M.; da Silva Almeida, H.; Duvoisin, S., Jr.; Pizarro Borges, L.E.; Teixeira Machado, N. Production of Fuel-Like Fractions by Fractional Distillation of Bio-Oil from Açaí (Euterpe oleracea Mart.) Seeds Pyrolysis. Energies 2021, 14, 3713. https://0-doi-org.brum.beds.ac.uk/10.3390/en14133713
Rocha de Castro DA, da Silva Ribeiro HJ, Hamoy Guerreiro LH, Pinto Bernar L, Jonatan Bremer S, Costa Santo M, da Silva Almeida H, Duvoisin S Jr., Pizarro Borges LE, Teixeira Machado N. Production of Fuel-Like Fractions by Fractional Distillation of Bio-Oil from Açaí (Euterpe oleracea Mart.) Seeds Pyrolysis. Energies. 2021; 14(13):3713. https://0-doi-org.brum.beds.ac.uk/10.3390/en14133713
Chicago/Turabian StyleRocha de Castro, Douglas Alberto, Haroldo Jorge da Silva Ribeiro, Lauro Henrique Hamoy Guerreiro, Lucas Pinto Bernar, Sami Jonatan Bremer, Marcelo Costa Santo, Hélio da Silva Almeida, Sergio Duvoisin, Jr., Luiz Eduardo Pizarro Borges, and Nélio Teixeira Machado. 2021. "Production of Fuel-Like Fractions by Fractional Distillation of Bio-Oil from Açaí (Euterpe oleracea Mart.) Seeds Pyrolysis" Energies 14, no. 13: 3713. https://0-doi-org.brum.beds.ac.uk/10.3390/en14133713