Persistent Organic Pollutants (POPs) in Fish Consumed by the Indigenous Peoples from Nenets Autonomous Okrug
Abstract
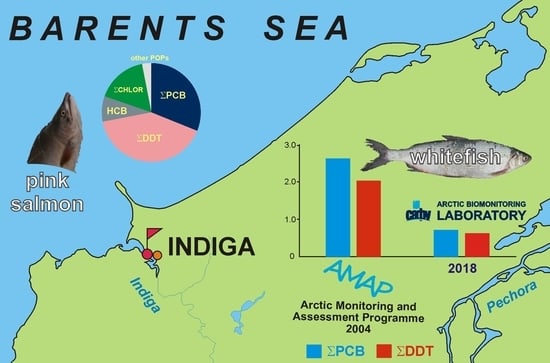
:1. Introduction
2. Materials and Methods
2.1. Sampling Strategy
2.2. Reagents and Materials
2.3. Determination of POP
2.4. Optimization of Gas Chromatography and Mass Spectrometry Parameters
2.5. Sample Preparation and Clean Up
2.6. Quality Control and Validation
2.7. Lipid Determination
2.8. Statistical Analysis
3. Results and Discussion
3.1. PCB Congeners
3.2. DDT Breakdown Products
3.3. Other POPs
3.4. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Wit, C.A.; Herzke, D.; Vorkamp, K. Brominated flame retardants in the Arctic environment—Trends and new candidates. Sci. Total Environ. 2010, 408, 2885–2918. [Google Scholar] [CrossRef]
- Braune, B.M.; Outridge, P.M.; Fisk, A.T.; Muir, D.C.G.; Helm, P.A.; Hobbs, K.; Hoekstra, P.F.; Kuzyk, Z.A.; Kwan, M.; Leteher, R.J.; et al. Persistent organic pollutants and mercury in marine biota of the Canadian Arctic: An overview of spatial and temporal trends. Sci. Total Environ. 2005, 351–352, 4–56. [Google Scholar] [CrossRef] [PubMed]
- Marques-Pinto, A.; Carvalho, D. Human infertility: Are endocrine disruptors to blame? Endocr. Connect. 2013, 2, 15–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Golding, J.; Emmett, P.; Iles-Caven, Y.; Steer, C.; Lingam, R. A Review of Environmental Contributions to Childhood Motor Skills. J. Child Neurol. 2013, 29, 1531–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organisation (WHO). Agrochemicals, Health and Environment: Directory of Resources. Available online: http://www.who.int/heli/risks/toxics/chemicalsdirectory/en/index1.html (accessed on 10 September 2019).
- Jurewicz, J.; Polańska, K.; Hanke, W. Chemical exposure early in life and the neurodevelopment of children—An overview of current epidemiological evidence. Ann. Agric. Environ. Med. 2013, 20, 465–486. [Google Scholar]
- Mughal, B.B.; Fini, J.-B.; Demeneix, B.A. Thyroid-disrupting chemicals and brain development: An update. Endocr. Connect. 2018, 7, 160–186. [Google Scholar] [CrossRef] [Green Version]
- Sorokina, T.Y. A national system of biological monitoring in the Russian Arctic as a tool for the implementation of the Stockholm Convention. Int. Environ. Agreem. Politics Law Econ. 2019, 19, 341–355. [Google Scholar] [CrossRef] [Green Version]
- AMAP. Biological Effects of Contaminants on Arctic Wildlife and Fish; Arctic Monitoring and Assessment Programme (AMAP): Tromsø, Norway, 2018. [Google Scholar]
- Abass, K.; Emelyanova, A.; Rautio, A. Temporal trends of contaminants in Arctic human populations. Environ. Sci. Pollut. Res. 2018, 25, 28834–28850. [Google Scholar] [CrossRef] [Green Version]
- Petrenya, N.; Dobrodeeva, L.; Brustad, M.; Bichkaeva, F.; Menshikova, E.; Lutfalieva, G.; Poletaeva, A.; Poletaeva, A.; Repina, V.; Cooper, M.; et al. Fish consumption and socio-economic factors among residents of Arkhangelsk city and the rural Nenets autonomous area. Int. J. Circumpolar Health 2011, 70, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Dudarev, A.; Yamin-Pasternak, S.; Pasternak, I.; Chupakhin, V. Traditional Diet and Environmental Contaminants in Coastal Chukotka I: Study Design and Dietary Patterns. Int. J. Environ. Res. Public Health 2019, 16, 702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosstat. The Diet of the Population [Ratsion Pitaniya Naseleniya]; Russian Federal State Statistics Service (Rosstat): Moscow, Russian, 2016.
- Uekusa, Y.; Takatsuki, S.; Tsutsumi, T.; Akiyama, H.; Matsuda, R.; Teshima, R.; Hachisuko, A.; Watanabe, T. Determination of polychlorinated biphenyls in marine fish obtained from tsunami-stricken areas of Japan. PLoS ONE 2017, 12, e0174961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manirakiza, P.; Covaci, A.; Nizigiymana, L.; Ntakimazi, G.; Schepens, P. Persistent chlorinated pesticides and polychlorinated biphenyls in selected fish species from Lake Tanganyika, Burundi, Africa. Environ. Pollut. 2002, 117, 447–455. [Google Scholar] [CrossRef]
- Muir, D.; Savinova, T.; Savinov, V.; Alexeeva, L.; Potelov, V.; Svetochev, V. Bioaccumulation of PCBs and chlorinated pesticides in seals, fishes and invertebrates from the b, Russia. Sci. Total Environ. 2003, 306, 111–131. [Google Scholar] [CrossRef]
- AMAP. Temporal Trends in Persistent Organic Pollutants in the Arctic; AMAP Assessment 2015; Arctic Monitoring and Assessment Programme (AMAP): Oslo, Norway, 2016. [Google Scholar]
- Stockholm Convention on Persistent Organic Pollutants Was Adopted on 22 May 2001 in Stockholm, Sweden. Available online: http://chm.pops.int/Portals/0/Repository/convention_text/UNEP-POPS-COP-CONVTEXT-FULL.English.PDF (accessed on 19 September 2019).
- Letcher, R.J.; Bustnes, J.O.; Dietz, R.; Jenssen, B.M.; Jørgensen, E.H.; Sonne, C.; Verrault, J.; Vijayan, M.; Gabrielsen, G.W. Exposure and effects assessment of persistent organohalogen contaminants in arctic wildlife and fish. Sci. Total Environ. 2010, 408, 2995–3043. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, J.S.A.; Delgado, I.F.; Paumgartten, F.J.R. Glyphosate and adverse pregnancy outcomes, a systematic review of observational studies. BMC Public Health 2016, 16, 472. [Google Scholar] [CrossRef] [Green Version]
- Sobolev, N.; Aksenov, A.; Sorokina, T.; Chashchin, V.; Ellingsen, D.; Nieboer, E.; Varakina, Y.; Veselkina, E.; Kotsur, D.; Thomassen, Y. Essential and non-essential trace elements in fish consumed by indigenous peoples of the European Russian Arctic. Environ. Pollut. 2019, 253, 966–973. [Google Scholar] [CrossRef]
- Sobolev, N.; Nieboer, E.; Aksenov, A.; Sorokina, T.; Chashchin, V.; Ellingsen, D.G.; Varakina, Y.; Plakhina, E.; Kotsur, D.; Kosheleva, A.; et al. Concentration dataset for 4 essential and 5 non-essential elements in fish collected in Arctic and sub-Arctic territories of the Nenets Autonomous and Arkhangelsk regions of Russia. Data Brief 2019, 27, 104631. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Tsygankov, V.Y.; Boyarova, M.D. Sample preparation method for the determination of organochlorine pesticides in aquatic organisms by gas chromatography. Achieve Life Sci. 2015, 9, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Bonito, L.T.; Hamdoun, A.; Sandin, S.A. Evaluation of the global impacts of mitigation on persistent, bioaccumulative and toxic pollutants in marine fish. PeerJ 2016, 4, e1573. [Google Scholar] [CrossRef] [PubMed]
- Perugini, M.; Cavaliere, M.; Giammarino, A.; Mazzone, P.; Olivieri, V.; Amorena, M. Levels of polychlorinated biphenyls and organochlorine pesticides in some edible marine organisms from the Central Adriatic Sea. Chemosphere 2004, 57, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, Y.; Li, A.; Zhang, Q.; Jing, C.; Wang, T.; Li, Y.; Jiang, G. Organochlorine pesticides and PCBs in fish from lakes of the Tibetan Plateau and the implications. Environ. Pollut. 2010, 158, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- AMAP. Persistent Toxic Substances, Food Security and Indigenous Peoples of the Russian North. Final Report; Arctic Monitoring and Assessment Programme (AMAP): Oslo, Norway, 2004. [Google Scholar]
- Tsygankova, V.; Lukyanovaa, O.; Boyarovaa, M.; Gumovskiya, A.; Donetsa, M.; Lyakha, V.; Korchagina, V.; Prikhodkoa, Y. Organochlorine pesticides in commercial Pacific salmon in the Russian Far Eastern seas: Food safety and human health risk assessment. Mar. Pollut. Bull. 2019, 140, 503–508. [Google Scholar] [CrossRef]
- Cabrerizo, A.; Muir, D.C.G.; Köck, G.; Iqaluk, D.; Wang, X. Climatic Influence on Temporal Trends of Polychlorinated Biphenyls and Organochlorine Pesticides in Landlocked Char from Lakes in the Canadian High Arctic. Environ. Sci. Technol. 2018, 52, 10380–10390. [Google Scholar] [CrossRef]
- Storelli, M.M.; Losada, S.; Marcotrigiano, G.O.; Roosens, L.; Barone, G.; Neels, H.; Covaci, A. Polychlorinated biphenyl and organochlorine pesticide contamination signatures in deep-sea fish from the Mediterranean Sea. Environ. Res. 2009, 109, 851–856. [Google Scholar] [CrossRef]






| Fish Species | n | Weight (kg) | Age (Year) | Lipid (%) | |||
|---|---|---|---|---|---|---|---|
| AM | Min–Max | AM | Min–Max | AM | Min–Max | ||
| Arctic char (Salvelinus alpinus) | 10 | 0.73 | 0.47–1.05 | 4.5 | 3.0–6.5 | 1.37 | 0.16–3.30 |
| Pink salmon (Oncorhynchus gorbuscha) | 12 | 1.07 | 0.80–1.64 | 1+ * | 1–2 | 2.93 | 2.15–4.73 |
| Navaga (Eleginus nawaga) | 10 | 0.22 | 0.13–0.38 | 4.5 | 3.0–6.5 | 0.51 | 0.27–1.03 |
| Humpback whitefish (Coregonus pidschian) | 11 | 0.44 | 0.38–0.57 | 7.0 | 5.0–10.0 | 0.17 | 0.02–0.46 |
| Northern pike (Esox lucius) | 8 | 3.45 | 1.07–6.53 | 5.0 | 1.5–8.5 | 0.10 | 0.02–0.54 |
| Analytes, ng/g (ww) | Limit of Quantification (LOQ) | Limit of Detection (LOD) | Arctic Char (N = 10) | Pink Salmon (N = 12) | Navaga (N = 10) | Humpback Whitefish (N = 11) | Northern Pike (N = 8) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AM | Min–Max | AM | Min–Max | AM | Min–Max | AM | Min–Max | AM | Min–Max | |||
| ∑PCB8 | 0.03 | 0.008 | 1.58 | 0.56–2.44 | 1.54 | 0.41–2.89 | 1.24 | 0.46–2.59 | 0.72 | 0.34–1.45 | 0.32 | 0.09–0.50 |
| DL-PCB * | 0.19 | 0.11–0.25 | 0.21 | 0.02–0.38 | 0.13 | 0–0.21 | - | - | - | - | ||
| NDL-PCB ** | 1.39 | 0.45–2.20 | 1.20 | 0.40–1.97 | 1.10 | 0.38–2.38 | 0.72 | 0.34–1.45 | 0.43 | 0–0.5 | ||
| ∑DDT | 0.03 | 0.009 | 0.66 | 0.33–1.11 | 2.04 | 0.50–3.95 | 0.55 | 0.18–0.88 | 0.63 | 0.27–1.25 | 0.08 | 0.03–0.15 |
| HCB | 0.03 | 0.010 | 0.31 | 0.04–0.85 | 0.40 | 0.12–0.63 | 0.39 | 0.14–0.70 | 0.28 | 0.02–0.95 | 0.13 | 0.03–0.29 |
| ∑Tetrachlorobenzene | 0.004 | 0.001 | 0.02 | 0.01–0.04 | 0.02 | 0.01–0.03 | 0.02 | 0.01–0.06 | 0.17 | 0.09–0.35 | <LOD | |
| Mirex | 0.01 | 0.003 | 0.05 | 0.04–0.08 | 0.13 | 0.10–0.16 | 0.04 | 0.01–0.06 | 0.06 | 0.01–0.33 | <LOD | |
| cis-Nonachlor | 0.12 | 0.040 | 0.07 | 0.06–0.10 | 0.17 | 0.06–0.30 | 0.08 | 0.06–0.20 | <LOD | <LOD | ||
| trans-Nonachlor | 0.08 | 0.027 | 0.13 | 0.04–0.31 | 0.40 | 0.12–0.71 | 0.07 | 0.03–0.12 | <LOD | <LOD | ||
| trans-Chlordane | 0.04 | 0.013 | 0.03 | 0.02–0.06 | 0.10 | 0.04–0.16 | 0.03 | 0.02–0.04 | <LOD | <LOD | ||
| cis-Chlordane(ng/g) | 0.04 | 0.013 | 0.05 | 0.02–0.09 | 0.17 | 0.06–0.29 | 0.04 | 0.02–0.09 | <LOD | <LOD | ||
| β-Heptachlor epoxide | 0.03 | 0.010 | <LOD | <LOD | <LOD | <LOD | <LOD | |||||
| Aldrin | 0.04 | 0.013 | <LOD | <LOD | <LOD | <LOD | <LOD | |||||
| Heptachlor | 0.05 | 0.016 | <LOD | <LOD | <LOD | <LOD | <LOD | |||||
| PCB 52 | PCB 101 | PCB 105 | PCB 118,123 | PCB 128 | PCB 153 | ∑PCB | ∑DDT | HCB | cis-Nonachlor | trans-Nonachlor | trans-Chlordane | cis-Chlordane | Mirex | ∑Tetrachloro-Benzene | Lipid | Weight | Age | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCB#28 | 0.461 ** | 0.424 ** | 0.330 | 0.246 | 0.384 * | 0.406 ** | 0.400 ** | 0.215 | 0.338 * | 0.056 | 0.315 | 0.231 | 0.202 | −0.156 | −0.090 | 0.054 | 0.135 | 0.162 |
| PCB#52 | 0.637 ** | 0.654 ** | 0.664 ** | 0.726 ** | 0.338 * | 0.644 ** | 0.695 ** | 0.463 ** | 0.577 ** | 0.831 ** | 0.784 ** | 0.738 ** | 0.332 * | −0.110 | 0.550 ** | 0.439 ** | −0.174 | |
| PCB#101 | 0.459 * | 0.673 ** | 0.680 ** | 0.486 ** | 0.840 ** | 0.536 ** | 0.392 ** | 0.335 | 0.558 ** | 0.510 ** | 0.423 * | 0.490 ** | −0.499 ** | 0.658 ** | 0.529 ** | −0.379 * | ||
| PCB#105 | 0.589 ** | 0.656 ** | 0.522 * | 0.646 ** | 0.542 ** | 0.357 | 0.521 * | 0.541 ** | 0.509 * | 0.523 * | 0.326 | 0.138 | 0.445 * | 0.449 * | −0.014 | |||
| PCB#118,123 | 0.791 ** | 0.706 ** | 0.837 ** | 0.836 ** | 0.727 ** | 0.650 ** | 0.658 ** | 0.673 ** | 0.608 ** | 0.524 ** | 0.348 | 0.590 ** | 0.403 * | −0.191 | ||||
| PCB#128 | 0.486 ** | 0.825 ** | 0.725 ** | 0.388 * | 0.689 ** | 0.816 ** | 0.695 ** | 0.694 ** | 0.358 * | −0.188 | 0.520 ** | 0.244 | −0.286 | |||||
| PCB#153 | 0.763 ** | 0.413 ** | 0.537 ** | 0.232 | 0.124 | 0.098 | 0.058 | 0.063 | 0.017 | 0.107 | −0.102 | 0.188 | ||||||
| ∑PCB | 0.779 ** | 0.663 ** | 0.527 ** | 0.590 ** | 0.508 ** | 0.471 ** | 0.328 * | −0.379 * | 0.638 ** | −0.072 | −0.280 * | |||||||
| ∑DDT | 0.596 ** | 0.821 ** | 0.914 ** | 0.901 ** | 0.864 ** | 0.603 ** | −0.135 | 0.724 ** | 0.025 | −0.360 ** | ||||||||
| HCB | 0.461 ** | 0.274 | 0.434 * | 0.390 * | 0.171 | 0.073 | 0.543 ** | −0.127 | −0.188 | |||||||||
| cis-Nonachlor | 0.773 ** | 0.842 ** | 0.871 ** | 0.667 ** | 0.066 | 0.654 ** | 0.605 ** | −0.441 * | ||||||||||
| trans-Nonachlor | 0.901 ** | 0.883 ** | 0.695 ** | −0.146 | 0.733 ** | 0.749 ** | −0.603 ** | |||||||||||
| trans-Chlordane | 0.941 ** | 0.808 ** | 0.038 | 0.866 ** | 0.799 ** | −0.508 ** | ||||||||||||
| cis-Chlordane | 0.749 ** | −0.036 | 0.800 ** | 0.746 ** | −0.484 ** | |||||||||||||
| Mirex | −0.315 * | 0.708 ** | 0.615 ** | −0.651 ** | ||||||||||||||
| ∑Tetrachloro-benzene | −0.499 ** | −0.269 | 0.611 ** | |||||||||||||||
| Lipid | 0.107 | −0.616 ** | ||||||||||||||||
| Weight | −0.212 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakhmanov, D.; Varakina, Y.; Aksenov, A.; Sorokina, T.; Sobolev, N.; Kotsur, D.; Plakhina, E.; Chashchin, V.; Thomassen, Y. Persistent Organic Pollutants (POPs) in Fish Consumed by the Indigenous Peoples from Nenets Autonomous Okrug. Environments 2020, 7, 3. https://0-doi-org.brum.beds.ac.uk/10.3390/environments7010003
Lakhmanov D, Varakina Y, Aksenov A, Sorokina T, Sobolev N, Kotsur D, Plakhina E, Chashchin V, Thomassen Y. Persistent Organic Pollutants (POPs) in Fish Consumed by the Indigenous Peoples from Nenets Autonomous Okrug. Environments. 2020; 7(1):3. https://0-doi-org.brum.beds.ac.uk/10.3390/environments7010003
Chicago/Turabian StyleLakhmanov, Dmitry, Yulia Varakina, Andrey Aksenov, Tatiana Sorokina, Nikita Sobolev, Dmitry Kotsur, Elena Plakhina, Valery Chashchin, and Yngvar Thomassen. 2020. "Persistent Organic Pollutants (POPs) in Fish Consumed by the Indigenous Peoples from Nenets Autonomous Okrug" Environments 7, no. 1: 3. https://0-doi-org.brum.beds.ac.uk/10.3390/environments7010003