Monitoring Arsenic Species Content in Seaweeds Produced off the Southern Coast of Korea and Its Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
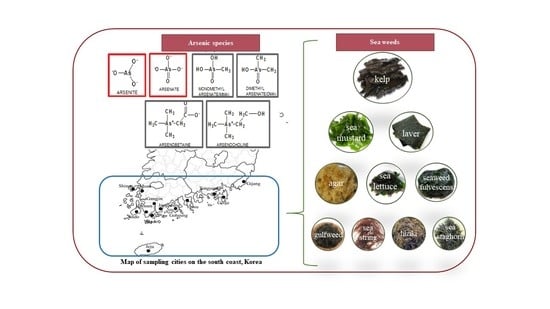
2.2. Sample Collection and Preparation
2.3. Instruments
2.4. Determination of Total As
2.5. Determination of As Species
2.6. Validation
2.7. Risk Assessment
2.8. Statistical Analysis
3. Results and Discussion
3.1. Validation Results of Analytical Methods
3.2. Determination of Total As and As Species in Seaweed
3.3. Correlation Analysis between Total Arsenic and Inorganic Arsenic
3.4. Clustering Analysis for Grouping
3.5. Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Devesa, V.; Martinez, A.; Suner, M.; Benito, V.; Vélez, D.; Montoro, R. Kinetic study of transformations of arsenic species during heat treatment. J. Agric. Food Chem. 2001, 49, 2267–2271. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. The role of selenium in arsenic and Cadmium toxicity: An updated review of scientific literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef] [Green Version]
- Styblo, M.; Del Razo, L.M.; Vega, L.; Germolec, D.R.; LeCluyse, E.L.; Hamilton, G.A.; Reed, W.; Wang, C.; Cullen, W.R.; Thomas, D.J. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch. Toxicol. 2000, 74, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Arnold, L.L.; Beck, B.D.; Lewis, A.S.; Eldan, M. Evaluation of the carcinogenicity of inorganic arsenic. Crit. Rev. Toxicol. 2013, 43, 711–752. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.; Chu, H.M.; How, S.; Fong, J.; Lin, C.; Yeh, S. Prevalence of skin cancer in an endemic area of chronic arsenicism in Taiwan. J. Natl. Cancer Inst. 1968, 40, 453–463. [Google Scholar]
- IARC. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42; IARC: Lyon, France, 1987. [Google Scholar]
- Rousseau, M.-C.; Straif, K.; Siemiatycki, J. IARC carcinogen update. Environ. Health Perspect. 2005, 113, A580–A581. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Lee, H.; Yoon, H.O.; Woo, N.C. Kinetics of dimethylated thioarsenicals and the formation of highly toxic dimethylmonothioarsinic acid in environment. Environ. Sci. Technol. 2016, 50, 11637–11645. [Google Scholar] [CrossRef] [PubMed]
- Luvonga, C.; Rimmer, C.A.; Yu, L.L.; Lee, S.B. Organoarsenicals in Seafood: Occurrence, Dietary Exposure, Toxicity, and Risk Assessment Considerations—A Review. J. Agric. Food Chem. 2020, 68, 943–960. [Google Scholar] [CrossRef]
- IARC. A Review of Human Carcinogens: Arsenic, Metals, Fibres, and Dusts; IARC: Lyon, France, 2012; Volume 100C. [Google Scholar]
- Cubadda, F.; Jackson, B.P.; Cottingham, K.L.; Van Horne, Y.O.; Kurzius-Spencer, M. Human exposure to dietary inorganic arsenic and other arsenic species: State of knowledge, gaps and uncertainties. Sci. Total Environ. 2017, 579, 1228–1239. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cravedi, J.-P.; Di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; Farmer, P. Scientific Opinion on Arsenic in Food EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA J. 2009, 7, 1–198. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives; Weed Control in Organic Farming; World Health Organization. Safety Evaluation of Certain Contaminants in Food; World Health Organization: Geneva, Switzerland, 2006; pp. 1–753. [Google Scholar]
- Chung, I.K.; Beardall, J.; Mehta, S.; Sahoo, D.; Stojkovic, S. Using marine macroalgae for carbon sequestration: A critical appraisal. J. Appl. Phycol. 2011, 23, 877–886. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Hwang, Y.; Park, S.; Park, G.; Choi, S.; Kim, M. Total arsenic, mercury, lead, and cadmium contents in edible dried seaweed in Korea. Food Addit. Contam. Part B 2010, 3, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.-J.; Jiang, S.-J. Application of HPLC-ICP-MS and HPLC-ESI-MS procedures for arsenic speciation in seaweeds. J. Agric. Food Chem. 2012, 60, 2083–2089. [Google Scholar] [CrossRef]
- Maher, W.A. Inorganic Arsenic in Marine Organisms. Mar. Pollut. Bull. 1983, 14, 308–310. [Google Scholar] [CrossRef]
- Shibata, Y.; Sekiguchi, M.; Otsuki, A.; Morita, M. Arsenic Compounds in Zoo-and Phyto-plankton of Marine Origin. Appl. Organomet. Chem. 1996, 10, 713–719. [Google Scholar] [CrossRef]
- Hirata, S.; Toshimitsu, H. Determination of arsenic species and arsenosugars in marine samples by HPLC–ICP–MS. Anal. Bioanal. Chem. 2005, 383, 454–460. [Google Scholar] [CrossRef]
- Rose, M.; Lewis, J.; Langford, N.; Baxter, M.; Origgi, S.; Barber, M.; MacBain, H.; Thomas, K. Arsenic in seaweed—Forms, concentration and dietary exposure. Food Chem. Toxicol. 2007, 45, 1263–1267. [Google Scholar] [CrossRef]
- Taylor, V.F.; Jackson, B.P. Concentrations and speciation of arsenic in New England seaweed species harvested for food and agriculture. Chemosphere 2016, 163, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Almela, C.; Clemente, M.J.; Vélez, D.; Montoro, R. Total arsenic, inorganic arsenic, lead and cadmium contents in edible seaweed sold in Spain. Food Chem. Toxicol. 2006, 44, 1901–1908. [Google Scholar] [CrossRef] [PubMed]
- Llorente-Mirandes, T.; Ruiz-Chancho, M.J.; Barbero, M.; Rubio, R.; López-Sánchez, J.F. Determination of water-soluble arsenic compounds in commercial edible seaweed by LC-ICPMS. J. Agric. Food Chem. 2011, 59, 12963–12968. [Google Scholar] [CrossRef] [PubMed]
- MOF. Fisheries Statistics on the Sale of Marine Products 2017. Available online: https://www.fips.go.kr/p/S020601/ (accessed on 5 November 2019).
- KHIDI. National Nutrition Statistics 2015. Available online: https://www.khidi.or.kr/kps/dhraStat/intro?menuId=MENU01650&year=2015#sub04 (accessed on 5 November 2019).
- Lee, S.G.; Lee, Y.S.; Cho, S.-Y.; Chung, M.-S.; Cho, M.; Kang, Y.; Kim, H.; Kim, D.; Lee, K.-W. Monitoring of arsenic contents in domestic rice and human risk assessment for daily intake of inorganic arsenic in Korea. J. Food Compost. Anal. 2018, 69, 25–32. [Google Scholar] [CrossRef]
- MFDS. Korean Food Standard Codex Working Manual on Heavy Metals Analysis. Available online: http://www.foodsafetykorea.go.kr/foodcode/01_03.jsp?idx=12042 (accessed on 5 November 2019).
- MFDS. Manual for Testing Inorganic Arsenic in Food; MFDS: Chungcheongbuk-do, Korea, 2018; pp. 1–39. [Google Scholar]
- KCDC. The seventh Korea National Health and Nutrition Examination Survey (KNHA NES VII-1); Korea Centers for Disease Control and Prevention: Sejong, Korea, 2018. [Google Scholar]
- NHIS. Health Examination Statistics. Available online: http://kosis.kr/statHtml/statHtml.do?orgId=350&tblId=DT_35007_N132&vw_cd=MT_ZTITLE&list_id=350_35007_A007&seqNo=&lang_mode=ko&language=kor&obj_var_id=&itm_id=&conn_path=MT_ZTITLE (accessed on 5 November 2019).
- Norman, J.; Pickford, C.; Sanders, T.; Waller, M. Human intake of arsenic and iodine from seaweed-based food supplements and health foods available in the UK. Food Addit. Contam. 1988, 5, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, K.N.; Inoue, K.; Ohto, K.; Hayashida, T. Adsorption study of metal ions onto crosslinked seaweed Laminaria japonica. Bioresour. Technol. 2008, 99, 32–37. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Sosa-Hernández, J.E.; Raza, A.; Nabeel, F.; Iqbal, H. Biosorption: An interplay between marine algae and potentially toxic elements—A review. Mar. Drugs 2018, 16, 65. [Google Scholar] [CrossRef] [Green Version]
- Caumette, G.; Koch, I.; Reimer, K. Arsenobetaine formation in plankton: A review of studies at the base of the aquatic food chain. J. Environ. Monit. 2012, 14, 2841–2853. [Google Scholar] [CrossRef]
- Katayama, M.; Sugawa-Katayama, Y.; Murakami, K. Do the tissue concentrations of accumulated arsenic, calcium, iron, magnesium, manganese, potassium and zinc become niform throughout the Hijiki plant body with growth. Trace Nutr. Res. 2014, 31, 51–58. [Google Scholar]
- McSheehy, S.; Pohl, P.; Vélez, D.; Szpunar, J. Multidimensional liquid chromatography with parallel ICP MS and electrospray MS/MS detection as a tool for the characterization of arsenic species in algae. Anal. Bioanal. Chem. 2002, 372, 457–466. [Google Scholar] [CrossRef]
- Sand, S.; Bjerselius, R.; Busk, L.; Eneroth, H.; Färnstrand, J.S.; Lindqvist, R. The Risk Thermometer—A Tool for Risk Comparison. National Food Agency (Livsmedelsverket) Home Page. Available online: https://www.Livsmedelsverket.se/globalassets/rapportter/2015/the-risk-thermometer.pdfRapport (accessed on 9 November 2019).
- Yokoi, K.; Konomi, A. Toxicity of so-called edible hijiki seaweed (Sargassum fusiforme) containing inorganic arsenic. Regul. Toxicol. Pharmacol. 2012, 63, 291–297. [Google Scholar] [CrossRef]



| Method Detection Limit | Interday Precision and Recovery | ||||
|---|---|---|---|---|---|
| Arsenic Species 1 | R2 | LOD 2 (µg kg−1) | LOQ 3 (µg kg−1) | RSD 4 (%) | Rec 5 (%) |
| tAs | 0.9999 | 0.547 | 1.74 | 2.2 | 103.6 |
| AsⅤ | 0.9999 | 0.773 | 2.46 | 5.1 | 99 |
| AsⅢ | 0.9998 | 0.403 | 1.28 | ||
| MMA | 0.9999 | 0.593 | 1.89 | ||
| DMA | 0.9999 | 0.622 | 1.98 | ||
| AsB | 0.9999 | 0.535 | 1.70 | ||
| AsC | 0.9999 | 0.384 | 1.22 | ||
| Seaweed Categories (n) 1 | tAs (mg kg−1) | Concentration of each Arsenic Species (mg kg−1) | |||||
|---|---|---|---|---|---|---|---|
| AsV | AsIII | MMA | DMA | AsB | AsC | ||
| Kelp | 53.6 ± 14.6 2 | 0.163 ± 0.256 | 0.103 ± 0.080 | 0.113 ± 0.047 | 0.636 ± 0.285 | 0.834 ± 0.692 | 0.466 ± 0.507 |
| (n = 32) | (100) 3 | (69) | (72) | (91) | (97) | (75) | (94) |
| Sea mustard | 51.0 ± 15.8 | 0.036 ± 0.054 | 0.010 ± 0.023 | 0.011 ± 0.030 | 0.316 ± 0.302 | 0.179 ± 0.364 | 0.060 ± 0.160 |
| (n = 25) | (100) | (32) | (16) | (12) | (96) | (40) | (40) |
| Laver | 26.9 ± 5.3 | 0.064 ± 0.131 | 0.094 ± 0.061 | 0.046 ± 0.052 | 0.299 ± 0.133 | 0.034 ± 0.048 | 0.702 ± 1.518 |
| (n = 25) | (100) | (36) | (84) | (48) | (100) | (36) | (100) |
| Agar | 1.34 ± 1.53 | 0.109 ± 0.182 | 0.025 ± 0.044 | 0.037 ± 0.065 | 0.083 ± 0.111 | <LOD | 0.025 ± 1.035 |
| (n = 20) | (45) | (35) | (25) | (25) | (50) | (0) | (20) |
| Sea lettuce | 15.6 ± 11.8 | 0.085 ± 0.125 | 0.056 ± 0.092 | 0.036 ± 0.080 | 0.103 ± 0.157 | 0.006 ± 0.026 | 0.214 ± 0.282 |
| (n = 20) | (100) | (45) | (30) | (20) | (40) | (5) | (40) |
| Seaweed fulvescens | 5.54 ± 2.00 | 0.019 ± 0.053 | 0.014 ± 0.032 | 0.066 ± 0.057 | 0.035 ± 0.056 | 0.024 ± 0.045 | 0.004 ± 0.018 |
| (n = 17) | (100) | (12) | (18) | (59) | (30) | (24) | (6) |
| Gulfweed | 97.4 ± 26.0 | 53.6 ± 14.8 | 2.09 ± 1.57 | <LOD 4 | 1.45 ± 0.82 | <LOD | <LOD |
| (n = 12) | (100) | (100) | (75) | (0) | (100) | (0) | (0) |
| Sea staghorn | 11.8 ± 5.3 | 0.258 ± 0.115 | 0.201 ± 0.103 | 0.020 ± 0.071 | 0.282 ± 0.120 | 0.232 ± 0.135 | 0.414 ± 1.32 |
| (n = 13) | (100) | (100) | (100) | (8) | (100) | (100) | (46) |
| Sea string | 9.15 ± 5.20 | 0.144 ± 0.158 | 0.066 ± 0.091 | <LOD | 0.509 ± 0.954 | 0.029 ± 0.083 | 0.281 ± 0.369 |
| (n = 8) | (100) | (75) | (38) | (0) | (63) | (13) | (63) |
| Hijiki | 145 ± 61 | 93.2 ± 47.6 | 3.99 ± 3.55 | <LOD | 2.02 ± 1.75 | <LOD | <LOD |
| (n = 8) | (100) | (100) | (75) | (0) | (75) | (0) | (0) |
| Species | Trade Name | Country | n 1 | tAs | iAs | As(V) | As(III) | MMA | DMA | AsB | AsC | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Laminaria digitata | Kelp | New England | 6 | 57.77 | 8.32 | - | - | 0.06 | 0.97 | ND | - | [23] |
| Saccharina latissima | Kelp | New England | 1 | 56.29 | ND 2 | - | - | ND | 0.39 | ND | - | [23] |
| Laminaria sp. | Kelp | Spain | 1 | 39.6 | 0.473 | - | - | - | - | - | - | [23] |
| Laminaria sp. | Kelp | Japan | 1 | 48.3 | 0.145 | - | - | - | - | - | - | [23] |
| Laminaria japonica | Kelp | Japan | 2 | 110 | 0.838 | - | - | - | - | - | - | [23] |
| Laminaria digitata | Kelp | - | 1 | 65.7 | 0.251 | - | - | - | - | - | - | [23] |
| Alaria esculenta | Sea mustard | New England | 4 | 34.46 | 0.03 | - | - | ND | 0.47 | ND | - | [24] |
| Undaria pinnatifida | Sea mustard | Japan | 3 | 42.7 | ND | - | - | - | - | - | - | [23] |
| Undaria pinnatifida | Sea mustard | Spain | 3 | 35.5 | 0.586 | - | - | - | - | - | - | [23] |
| Undaria pinnatifida | Sea mustard | Korea | 1 | 46 | 1.06 | - | - | - | - | - | - | [23] |
| Porphyra umbilicalus | Laver | New England | 1 | 20.73 | 0.12 | - | - | 0.05 | 0.25 | ND | - | [24] |
| Porphyra tenera | Laver | Japan | 2 | 23.65 | 0.224 | - | - | - | - | - | - | [23] |
| Porphyra umbilicalis | Laver | Spain | 1 | 34.5 | 0.239 | - | - | - | - | - | - | [23] |
| Porphyra sp. | Laver | Japan | 1 | 32.7 | 0.189 | - | - | - | - | - | - | [23] |
| Porphyra sp. | Laver | Spain | 1 | 24.3 | 0.383 | - | - | - | - | - | - | [23] |
| Porphyra sp. | Laver | South Korea | 3 | 20.9 | 0.141 | - | - | - | - | - | - | [23] |
| Porphyra sp. | Laver | China | 2 | 50 | 0.313 | - | - | - | - | - | - | [23] |
| Ulva lactuca | Sea lettuce | New England | 2 | 4.74 | 0.075 | - | - | ND | 0.05 | 0.04 | - | [24] |
| Ulva prolifera | Sea lettuce | New England | 1 | 14.65 | 0.12 | - | - | ND | 0.04 | ND | - | [24] |
| Enteromorpha sp. | Sea lettuce | - | 1 | 2.15 | 0.346 | - | - | - | - | - | - | [23] |
| Ulva pertusa | Sea lettuce | - | 1 | 3.24 | 0.268 | - | - | - | - | - | - | [23] |
| Chondrus crispus | Sea string | New England | 3 | 10.12 | 0.07 | - | - | ND | 0.05 | 0.04 | - | [24] |
| Graciaria vermiculophylla | Sea string | New England | 1 | 11.8 | 0.23 | - | - | ND | 0.04 | ND | - | [24] |
| Chondrus crispus | Sea string | Spain | 2 | 14.1 | 0.599 | - | - | - | - | - | - | [23] |
| Hizikia fusiforme | Hijiki | Japan | 9 | 109.6 | 73.5 | - | - | - | - | - | - | [23] |
| Sargassum fulvellum | Gulfweed | - | 1 | 110.3 | 69.9 | 69.9 | ND | ND | 2.1 | ND | ND | [25] |
| Consumption Data | Margin of Exposure (MOE) 3 | |||
|---|---|---|---|---|
| Exposure Group | Seaweed Species | Daily Consumption (g day−1) 1 | iAs Intake (μg kg−1 day−1) 2 | BMDL01/iAs Intake |
| General group 4 | ||||
| Kelp | 1.55 × 10−4 | 6.30 × 10−7 | 4.76 × 105 | |
| Sea mustard | 1.14 × 10−4 | 7.99 × 10−8 | 3.75 × 106 | |
| Laver | 1.66 × 10−4 | 4.04 × 10−7 | 7.43 × 105 | |
| Gulfweed | 2.90 × 10−7 | 2.48 × 10−7 | 1.21 × 106 | |
| Hijiki | 1.10 × 10−5 | 1.63 × 10−5 | 1.84 × 104 | |
| Seaweed consumer group 5 | ||||
| Kelp | 2.14 × 10−3 | 8.69 × 10−6 | 3.45 × 104 | |
| Sea mustard | 7.68 × 10−4 | 5.37 × 10−7 | 5.58 × 105 | |
| Laver | 4.85 × 10−4 | 1.18 × 10−6 | 2.55 × 105 | |
| Gulfweed | 5.18 × 10−4 | 4.42 × 10−4 | 6.79 × 102 | |
| Hijiki | 1.76 × 10−3 | 2.62 × 10−3 | 1.15 × 102 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-h.; Kim, J.; Noh, C.-H.; Choi, S.; Joo, Y.-S.; Lee, K.-W. Monitoring Arsenic Species Content in Seaweeds Produced off the Southern Coast of Korea and Its Risk Assessment. Environments 2020, 7, 68. https://0-doi-org.brum.beds.ac.uk/10.3390/environments7090068
Kim M-h, Kim J, Noh C-H, Choi S, Joo Y-S, Lee K-W. Monitoring Arsenic Species Content in Seaweeds Produced off the Southern Coast of Korea and Its Risk Assessment. Environments. 2020; 7(9):68. https://0-doi-org.brum.beds.ac.uk/10.3390/environments7090068
Chicago/Turabian StyleKim, Min-hyuk, Junseob Kim, Chang-Hyun Noh, Seogyeong Choi, Yong-Sung Joo, and Kwang-Won Lee. 2020. "Monitoring Arsenic Species Content in Seaweeds Produced off the Southern Coast of Korea and Its Risk Assessment" Environments 7, no. 9: 68. https://0-doi-org.brum.beds.ac.uk/10.3390/environments7090068