The Control of Fusarium Root Rot and Development of Coastal Pine (Pinus thunbergii Parl.) Seedlings in a Container Nursery by Use of Bacillus licheniformis MH48
Abstract
:1. Introduction
2. Materials and Methods
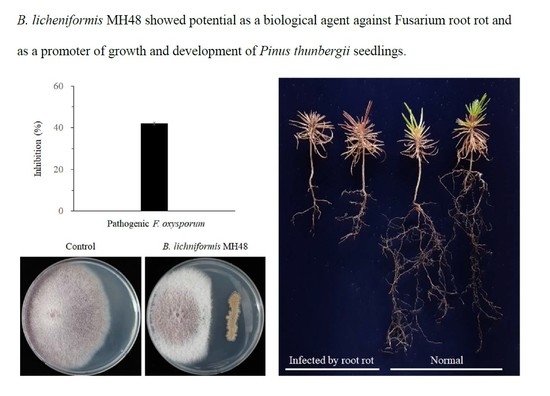
2.1. Antagonistic Activity of B. licheniformis MH48 against F. oxysporum
2.2. Production of the Defense-Related Lytic Enzymes by B. licheniformis MH48
2.3. Effect of Lytic Enzymes Produced from B. licheniformis MH48 on the Mycelial Morphology of F. oxysporum
2.4. Plant Material and Experimental Conditions
2.5. Growth Medium, Plant Sampling, and Measurement
2.6. Statistical Analysis
3. Results
3.1. Control of B. licheniformis MH48 against Fusarium Root Rot Disease
3.1.1. Production of Lytic Enzymes by B. licheniformis MH48
3.2. Effects of Inoculation with B. licheniformis MH48 on the Development of Coastal Pine Seedlings
3.2.1. Nutrient Contents of the Growing Media
3.2.2. Seedling Growth and Biomass
3.2.3. Seedling Nutrient Concentration and Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kudoh, T. Coastal disaster prevention forests in Japan. Jpn. Agric. Res. Q. 1983, 19, 55–58. [Google Scholar]
- Obase, K.; Cha, J.Y.; Lee, J.K.; Lee, S.Y.; Lee, J.H.; Chun, K.W. Ectomycorrhizal fungal communities associated with Pinus thunbergii in the eastern coastal pine forests of Korea. Mycorrhiza 2009, 20, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Obase, K.; Lee, J.K.; Lee, S.Y.; Chun, K.W. Diversity and community structure of ectomycorrhizal fungi in Pinus thunbergii coastal forests in the eastern region of Korea. Mycoscience 2011, 52, 383–391. [Google Scholar] [CrossRef]
- Zhu, J.; Gonda, Y.; Yu, L.; Li, F.; Yan, Q.; Sun, Y. Regeneration of a coastal pine (Pinus thunbergii Parl.) forest 11 years after thinning, Niigata, Japan. PLoS ONE 21012, 7, e47593. [Google Scholar] [CrossRef] [PubMed]
- Ewane, E.B.; Lee, J.H.; Lee, H.H. Eight-year monitoring of the height growth and survivorship of seedlings of Pinus thunbergii Parl. planted with sand fence and bush hedge protection in a coastal sandy environment in Korea. For. Sci. Technol. 2016, 12, 192–198. [Google Scholar] [CrossRef]
- Nateghi, R.; Bricker, J.D.; Guikema, S.D.; Bessho, A. Statistical analysis of the effectiveness of seawalls and coastal Forests in mitigating tsunami impacts in Iwate and Miyagi Prefectures. PLoS ONE 2016, 11, e0158375. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, W.J.; Slauson, K.M.; Carroll, C.R.; Kent, C.J.; Kudrna, D.G. Status of American martens in coastal forests of the Pacific states. J. Mammal. 2001, 82, 478–490. [Google Scholar] [CrossRef]
- Ensign, S.H.; Mallin, M.A. Stream water quality changes following timber harvest in a coastal plain swamp forest. Water Res. 2001, 35, 3381–3390. [Google Scholar] [CrossRef]
- FAO. Integrated Coastal Area Management and Agriculture, Forestry and Fisheries. In Issues, Perspectives, Policy and Planning Process for Integrated Coastal Area Management; Scialabba, N., Ed.; FAO: Rome, Italy, 1998; pp. 1–84. [Google Scholar]
- Tsakaldimi, M.; Zagas, T.; Tsitsoni, T.; Ganatsas, P. Root morphology, stem growth and field performance of seedlings of two Mediterranean evergreen oak species raised in different container types. Plant Soil 2005, 278, 85–93. [Google Scholar] [CrossRef]
- Wilson, E.; Vitols, K.C.; Park, A. Root characteristics and growth potential of container and bare-root seedlings of red oak (Quercus rubra L.) in Ontario, Canada. New For. 2007, 34, 163–176. [Google Scholar] [CrossRef]
- Tian, N.; Fang, S.; Yang, W.; Shang, X.; Fu, X. Influence of container type and growth medium on seedling growth and root morphology of Cyclocarya paliurus during nursery culture. Forests 2017, 8, 387. [Google Scholar] [CrossRef]
- Heiskanen, J. Effects of compost additive in sphagnum peat growing medium on Norway spruce container seedlings. New For. 2013, 44, 101–118. [Google Scholar] [CrossRef]
- Juntunen, M.L.; Hammar, T.; Rikala, R. Leaching of nitrogen and phosphorus during production of forest seedlings in containers. J. Environ. Qual. 2002, 31, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.M.A.; Eissa, M.F.M. Biofertilizers and their role in management of plant parasitic nematodes. A review. E3 J. Biotechnol. Pharm. Res. 2014, 5, 1–6. [Google Scholar]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Paungfoo-Lonhienne, C.; Yeoh, Y.K.; Kasinadhuni, N.R.P.; Lonhienne, T.G.A.; Robinson, N.; Hugenholtz, P.; Ragan, M.A.; Schmidt, S. Nitrogen fertilizer dose alters fungal communities in sugarcane soil and rhizosphere. Sci. Rep. 2015, 5, 8678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lilja, A.; Rikala, R. Effect of uninucleate Rhizoctonia on the survival of outplanted Scots pine and Norway spruce seedlings. For. Pathol. 2000, 30, 109–115. [Google Scholar] [CrossRef]
- Lilja, A.; Poteri, M.; Petaisto, R.L.; Rikala, R.; Kurkela, T.; Kasanen, R. Fungal diseases in forest nurseries in Finland. Silva Fenn. 2010, 44, 525–545. [Google Scholar] [CrossRef]
- Dar, G.H.; Beig, M.A.; Ahanger, F.A.; Nadeem, A.; Ganai, N.A.; Ahangar, M.A. Management of root rot caused by Rhizoctonia solani and Fusarium oxysporum in blue pine (Pinus wallichiana) through use of fungal antagonists. Asian J. Plant Pathol. 2011, 5, 62–74. [Google Scholar] [CrossRef]
- Shah, T.A.; Zargar, M.Y.; Dar, G.H. Interaction of Rhizoctonia solani with ectomycorrhizal inocula on deodar (Cedrus deodara). Appl. Biol. Res. 1999, 1, 103–107. [Google Scholar]
- Joshi, K.K.; Kumar, V.; Dubey, R.C.; Maheshwari, D.K. Effect of chemical fertilizer adaptive variants, Pseudomonas aeruginosa GRC2 and Azotobacter chroococcum AC1 on Macrophomena phaseolina causing charcoal rot of Brassica juncea. Korean J. Environ. Agric. 2006, 25, 228–235. [Google Scholar] [CrossRef]
- Jung, R.; Ahn, Y.S. Distribution of mercury concentrations in tree rings and surface soils adjacent to a phosphate fertilizer plant in southern Korea. Bull. Environ. Contam. Toxicol. 2017, 99, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Probanza, A.; Lucas García, J.A.; Ruiz Palomino, M.; Ramos, B.; Gutiérrez Mañero, F.Z. Pinus pinea L. seedling growth and bacterial rhizosphere structure after inoculation with PGPR Bacillus (B. licheniformis CECT 5106 and B. pumilus CECT 5105). Appl. Soil Ecol. 2002, 20, 75–84. [Google Scholar] [CrossRef]
- Park, H.G.; Jeong, M.H.; Ahn, Y.S. Inoculation with Bacillus licheniformis MH48 to improve Camellia japonica seedling development in coastal lands. Turk. J. Agric. For. 2007, 41, 381–388. [Google Scholar] [CrossRef]
- Park, H.G.; Lee, Y.S.; Kim, K.Y.; Park, Y.S.; Park, K.H.; Han, H.O.; Park, C.M.; Ahn, Y.S. Inoculation with Bacillus licheniformis MH48 promotes nutrient uptake in seedlings of the ornamental plant Camellia japonica grown in Korean reclaimed coastal lands. Hortic. Sci. Technol. 2017, 35, 11–20. [Google Scholar] [CrossRef]
- Esitken, A.; Karlidag, H.; Ercisli, S.; Turan, M.; Sahin, F. The effect of spraying a growth promoting bacterium on the yield, growth and nutrient element composition of leaves of apricot (Prunus armeniaca L. cv. Hacihaliloglu). Aust. J. Agric. Res. 2003, 54, 377–380. [Google Scholar] [CrossRef]
- Orhan, E.; Esitken, A.; Ercisli, S.; Turan, M.; Sahin, F. Effects of plant growth promoting rhizobacteria (PGPR) on yield, growth, and nutrient contents in organically growing raspberry. Sci. Hortic. 2006, 111, 38–43. [Google Scholar] [CrossRef]
- Barriuso, J.; Ramos Solano, B.; Santamaría, C.; Daza, A.; Gutiérrez Maňero, F.J. Effect of inoculation with putative plant growth-promoting rhizobacteria isolated from Pinus spp. on Pinus pinea growth, mycorrhization and rhizosphere microbial communities. J. Appl. Microbiol. 2008, 102, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Viterbo, A.; Ramot, O.; Chernin, L.; Chet, I. Significance of lytic enzymes from Trichoderma spp. in the biocontrol of fungal plant pathogens. Antonie Denderleeuw 2002, 81, 549–556. [Google Scholar] [CrossRef]
- Aktuganov, G.; Melentjev, A.; Galimzianova, N.; Khalikova, E.; Korpela, T.; Susi, P. Wide-range antifungal antagonism of Paenibacillus ehimensis IB-Xb and its dependence on chitinase and β-1,3-glucanase production. Can. J. Microbiol. 2008, 54, 577–587. [Google Scholar] [CrossRef]
- Mao, S.; Lee, S.J.; Hwangbo, H.; Kim, Y.W.; Park, K.H.; Chan, G.S.; Park, R.D.; Kim, K.Y. Isolation and characterization of antifungal substances from Burkholderia sp. culture broth. Curr. Microbiol. 2006, 53, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.S.; Jin, R.D.; Krishnan, H.B.; Lee, S.B.; Kim, K.Y. Biocontrol ability of Lysobacter antibioticus HS124 against Phytophthora blight is mediated by the production of 4-hydroxyphenylacetic acid and several lytic enzymes. Curr. Microbiol. 2009, 59, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Khabbaz, S.; Zhang, L.; Cáceres, L.; Sumarah, M.; Wang, A.; Abbasi, P. Characterisation of antagonistic Bacillus and Pseudomonas strains for biocontrol potential and suppression of damping-off and root rot diseases. Ann. Appl. Biol. 2015, 166, 456–471. [Google Scholar] [CrossRef]
- Jamal, Q.; Lee, Y.S.; Jeon, H.D.; Park, Y.S.; Kim, K.Y. Isolation and biocontrol potential of Bacillus amyloliquefaciens Y1 against fungal plant pathogens. Korean J. Soil Sci. Fertil. 2015, 48, 485–491. [Google Scholar] [CrossRef]
- Maung, C.E.H.; Choi, T.G.; Nam, H.H.; Kim, K.Y. Role of Bacillus amyloliquefaciens Y1 in the control of Fusarium wilt disease and growth promotion of tomato. Biocontrol Sci. Technol. 2017, 27, 1400–1415. [Google Scholar] [CrossRef]
- Zehnder, G.; Kloepper, J.; Yao, C.; Wei, G. Induction of systemic resistance in cucumber against cucumber beetles (Coleoptera, Chrysomelidae) by plant growth-promoting rhizobacteria. J. Econ. Entomol. 1997, 90, 391–396. [Google Scholar] [CrossRef]
- Jeong, M.H.; Yang, S.Y.; Lee, Y.S.; Ahn, Y.S.; Park, Y.S.; Han, H.R.; Kim, K.Y. Selection and characterization of Bacillus licheniformis MH48 for the biocontrol of pine wood nematode (Bursaphelenchus xylophilus). J. Korean For. Soc. 2015, 104, 512–518. [Google Scholar] [CrossRef]
- Lee, Y.S.; Nguyen, X.H.; Naing, K.W.; Park, Y.S.; Kim, K.Y. Role of lytic enzymes secreted by Lysobacter capsici YS1215 in the control of root-knot nematode of tomato plants. Indian J. Microbiol. 2015, 55, 74–80. [Google Scholar] [CrossRef]
- Jeong, M.H.; Lee, Y.S.; Cho, J.Y.; Ahn, Y.S.; Moon, J.H.; Hyun, H.N.; Cha, G.S.; Kim, K.Y. Isolation and characterization of metabolites from Bacillus licheniformis MH48 with antifungal activity against plant pathogens. Microb. Pathog. 2017, 110, 645–653. [Google Scholar] [CrossRef]
- Asiegbu, F.O.; Kacprzak, M.; Daniel, G.; Johansson, M.; Stenlid, J.; Manka, M. Biochemical interactions of conifer seedling roots with Fusarium spp. Can. J. Microbiol. 1999, 45, 923–935. [Google Scholar] [CrossRef]
- Chanway, C.P.; Holl, F.B. Influence of soil biota on Douglas fir (Pseudotsuga menziesii) seedling growth: The role of rhizosphere bacteria. Can. J. Bot. 1992, 70, 1025–1031. [Google Scholar] [CrossRef]
- Chanway, C.P.; Holl, F.B. Growth of outplanted lodgepole pine seedlings 1 year after inoculation with plant growth promoting rhizobacteria. For. Sci. 1994, 40, 238–246. [Google Scholar] [CrossRef]
- Chanway, C.P. Differential response of western hemlock from low and high elevations to inoculation with plant growthpromoting Bacillus polymyxa. Soil Biol. Biochem. 1995, 27, 767–775. [Google Scholar] [CrossRef]
- Leyval, C.; Berthelin, J. Rhizodeposition and net release of soluble organic compounds by pine and beech seedlings inoculated with rhizobacteria and ectomycorrhizal fungi. Biol. Fertil. Soils 1993, 15, 259–267. [Google Scholar] [CrossRef]
- Close, D.C.; Paterson, S.; Corkrey, R.; McArthur, C. Influences of seedling size, container type and mammal browsing on the establishment of Eucalyptus globulus in plantation forestry. New For. 2010, 39, 105–115. [Google Scholar] [CrossRef]
- South, D.B.; Mitchell, R.J. Determining the “optimum” slash pine seedling size for use with four levels of vegetation management on a flatwoods site in Georgia USA. Can. J. For. Res. 1999, 29, 1039–1046. [Google Scholar] [CrossRef]
- Cuesta, B.; Villar-Salvador, P.; Puértolas, J.; Jacobs, D.F.; Benayas, J.M.R. Why do large, nitrogen rich seedlings better resist stressful transplanting conditions? A physiological analysis in two functionally contrasting Mediterranean forest species. For. Ecol. Manag. 2010, 260, 71–78. [Google Scholar] [CrossRef]
- Skidmore, A.; Dickinson, C. Colony interactions and hyphal interference between Septoria nodorum and phylloplane fungi. Trans. Br. Mycol. Soc. 1976, 66, 57–64. [Google Scholar] [CrossRef]
- Lingappa, Y.; Lockwood, J. Chitin media for selective isolation and culture of Actinomycetes. Phytopathology 1962, 52, 317–323. [Google Scholar]
- Liang, Z.C.; Hseu, R.S.; Wang, H.H. Partial purification and characterization of a 1,3-β-d-glucanase from Ganoderma tsugae. J. Ind. Microbiol. 1995, 14, 5–9. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, Y.K.; Kim, Y.S.; Byoun, J.K.; Lee, S.W. Development of Soil Management Technique for Healthy Seedling Production in the Forest Nursery, 1st ed.; Korea Forest Research Institute: Seoul, Korea, 2012; pp. 57–80. (In Korean) [Google Scholar]
- Mulvaney, R.L. Nitrogen Inorganic Forms. In Methods of Soil Analysis: Part 3, Chemical Methods; Spark, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpoor, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]




| Treatment | Growth Media Nutrient Content (g kg−1) | Seedling Nutrient Concentration (%) | Seedling Nutrient Content (mg) | |||
|---|---|---|---|---|---|---|
| Total Nitrogen | Total Phosphorus | Total Nitrogen | Total Phosphorus | Total Nitrogen | Total Phosphorus | |
| Control | 0.21 ± 0.04 a | 0.05 ± 0.02 | 0.69 ± 0.15 c | 0.15 ± 0.05 b | 17.04 ± 5.07 b | 3.67 ± 1.36 b |
| Chemical fertilizer | 0.20 ± 0.02 a | 0.05 ± 0.01 | 1.41 ± 0.16 a | 0.23 ± 0.03 a | 46.62 ± 13.03 a | 7.65 ± 2.38 a |
| Bacterial inoculation | 0.15 ± 0.01 b | 0.06 ± 0.01 | 1.07 ± 0.15 b | 0.23 ± 0.03 a | 42.06 ± 12.21 a | 9.24 ± 2.81 a |
| Treatment | Seedling Growth | Seedling Biomass | |||||
|---|---|---|---|---|---|---|---|
| Shoot Length (cm) | Root Length (cm) | Total Height (cm) | Root Collar Diameter (mm) | Shoot Dry Mass (g) | Root Dry Mass (g) | Total Dry Mass (g) | |
| Control | 15.23 ± 3.23 c | 13.81 ± 2.15 b | 29.04 ± 4.18 b | 3.38 ± 0.32 c | 1.99 ± 0.34 c | 0.45 ± 0.14 b | 2.44 ± 0.45 c |
| Chemical fertilizer | 19.10 ± 2.38 b | 11.73 ± 1.96 c | 30.83 ± 2.36 b | 3.62 ± 0.34 b | 2.85 ± 0.48 b | 0.47 ± 0.12 b | 3.32 ± 0.55 b |
| Bacterial inoculation | 21.10 ± 3.86 a | 15.94 ± 1.96 a | 37.04 ± 4.85 a | 4.01 ± 0.48 a | 3.40 ± 0.56 a | 0.57 ± 0.12 a | 3.97 ± 0.64 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, S.-J.; Choub, V.; Kwon, J.-H.; Kim, D.-H.; Ahn, Y.-S. The Control of Fusarium Root Rot and Development of Coastal Pine (Pinus thunbergii Parl.) Seedlings in a Container Nursery by Use of Bacillus licheniformis MH48. Forests 2019, 10, 6. https://0-doi-org.brum.beds.ac.uk/10.3390/f10010006
Won S-J, Choub V, Kwon J-H, Kim D-H, Ahn Y-S. The Control of Fusarium Root Rot and Development of Coastal Pine (Pinus thunbergii Parl.) Seedlings in a Container Nursery by Use of Bacillus licheniformis MH48. Forests. 2019; 10(1):6. https://0-doi-org.brum.beds.ac.uk/10.3390/f10010006
Chicago/Turabian StyleWon, Sang-Jae, Vantha Choub, Jun-Hyeok Kwon, Dong-Hyun Kim, and Young-Sang Ahn. 2019. "The Control of Fusarium Root Rot and Development of Coastal Pine (Pinus thunbergii Parl.) Seedlings in a Container Nursery by Use of Bacillus licheniformis MH48" Forests 10, no. 1: 6. https://0-doi-org.brum.beds.ac.uk/10.3390/f10010006