The Complex Issue of Urban Trees—Stress Factor Accumulation and Ecological Service Possibilities
Abstract
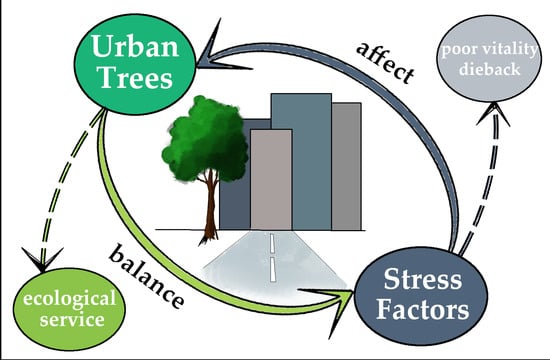
:1. Introduction
2. Abiotic Stress Factors in the City Area
2.1. Soil Degradation
2.1.1. Soil Compaction
2.1.2. Soil Drought
2.1.3. Extreme Soil Temperatures
2.1.4. Contamination, pH Changes, Nutrient Deficiency
2.2. Air Quality
2.2.1. Air Temperature
2.2.2. Air Pollution
2.3. Light Condition Disturbances
2.3.1. Inadequate Light Doses
2.3.2. Artificial Light Pollution
3. Tree Dieback in Urban Areas
4. Importance of Trees in Urban Ecosystems
4.1. Changes in Perception of Urban Trees Role in History
4.2. Social Benefits
4.3. Soil
4.4. Urban Microclimate and Air Pollution
4.5. Economic Benefits
5. Improper Management and Possible Disadvantages of Urban Trees (Disservices)
6. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations. World Urbanization Prospects: The 2014 Revision, Highlights; Department of Economic and Social Affairs, Population Division, United Nations: New York, NY, USA, 2014. [Google Scholar]
- Percival, G.C. Abiotic Stress. In Routledge Handbook of Urban Forestry; Ferrini, F., van den Bosch, C.C.K., Fini, A., Eds.; Taylor & Francis: Abingdon, UK, 2017. [Google Scholar]
- Jim, C.Y. Soil volume restrictions and urban soil design for trees in confined planting sites. J. Landsc. Arch. 2019, 14, 84–91. [Google Scholar] [CrossRef]
- Clark, J.R.; Kjelgren, R. Water as a limiting factor in the development of urban trees. J. Arboric. 1990, 16, 203–208. [Google Scholar]
- World Health Organization. Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide; World Health Organization: Copenhagen, Denmark, 2006. [Google Scholar]
- Li, D.H.W.; Wong, S.L. Daylighting and energy implications due to shading effects from nearby buildings. Appl. Energy 2007, 84, 1199–1209. [Google Scholar] [CrossRef]
- Xiao, R.; Su, S.; Zhang, Z.; Qi, J.; Jiang, D.; Wu, J. Dynamics of soil sealing and soil landscape patterns under rapid urbanization. Catena 2013, 109, 1–12. [Google Scholar] [CrossRef]
- Escobedo, F.J.; Kroeger, T.; Wagner, J.E. Urban forests and pollution mitigation: Analyzing ecosystem services and disservices. Environ. Pollut. 2011, 159, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Byrne, J.; Pickering, C. A systematic quantitative review of urban tree benefits, costs, and assessment methods across cities in different climatic zones. Urban For. Urban Green. 2012, 11, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Sæbø, A.; Borzan, Ž.; Ducatillion, C.; Hatzistathis, A.; Lagerström, T.; Supuka, J.; García-Valdecantos, J.L.; Rego, F.; Van Slycken, J. The selection of plant materials for street trees, park trees and urban woodland. In Urban Forests and Trees; Konijnendijk, C., Nilsson, K., Randrup, T., Schipperijn, J., Eds.; Springer: Berlin, Germany, 2005; pp. 257–280. [Google Scholar]
- Lavelle, P.; Spain, A.V. Soil Ecology, 2nd ed.; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Devigne, C.; Mouchon, P.; Vanhee, B. Impact of soil compaction on soil biodiversity—Does it matter in urban context? Urban Ecosyst. 2016, 19, 1163–1178. [Google Scholar] [CrossRef]
- Sandor, J.A.; Homburg, J.A. Anthropogenic Soil Change in Ancient and Traditional Agricultural Fields in Arid to Semiarid Regions of the Americas. J. Ethnobiol. 2017, 37, 196–217. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Zhu, L.; Zhou, D. POLSOIL: Research on soil pollution in China. Environ. Sci. Pollut. Res. 2018, 25, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Layman, R.M.; Day, S.D.; Mitchell, D.K.; Chen, Y.; Harris, J.R.; Daniels, W.L. Below ground matters: Urban soil rehabilitation increases tree canopy and speeds establishment. Urban For. Urban Green. 2016, 16, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Way, D.; Oren, R.; Kroner, Y. The space-time continuum: The effects of elevated CO2 and temperature on trees and the importance of scaling. Plant Cell Environ. 2015, 38, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; Abkenar, K.T.; Mohammadian, M.A.; Shabanian, N. Effects of dust on forest tree health in Zagros oak forests. Environ. Monit. Assess. 2017, 189, 549. [Google Scholar] [CrossRef] [PubMed]
- McGrath, D.; Henry, J. Organic amendments decrease bulk density and improve tree establishment and growth in roadside plantings. Urban For. Urban Green. 2016, 20, 120–127. [Google Scholar] [CrossRef]
- Scalenghe, R.; Marsan, F.A. The anthropogenic sealing of soils in urban areas. Landsc. Urban Plan. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Day, S.D.; Wiseman, P.E.; Dickinson, S.B.; Harris, J.R. The Root Ecology in the Urban Environment and Implications for a Sustainable Rhizosphere. Arboric. Urban For. 2010, 36, 193–204. [Google Scholar]
- Bengough, A.G.; McKenzie, B.; Hallett, P.; Valentine, T.A. Root elongation, water stress, and mechanical impedance: A review of limiting stresses and beneficial root tip traits. J. Exp. Bot. 2011, 62, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Day, S.D.; Bassuk, N.L. A review of the effects of soil compaction and amelioration treatments on landscape trees. J. Arboric. 1994, 20, 9–17. [Google Scholar]
- Materechera, S.A.; Dexter, A.R.; Alston, A.M. Penetration of very strong soils by seedling roots of different plant species. Plant Soil 1991, 135, 31–41. [Google Scholar] [CrossRef]
- Skinner, A.K.; Lunt, I.D.; Spooner, P.G.; McIntyre, S. The effect of soil compaction on germination and early growth ofEucalyptus albensand an exotic annual grass. Austral Ecol. 2009, 34, 698–704. [Google Scholar] [CrossRef]
- Randrup, T.; McPherson, E.; Costello, L. A review of tree root conflicts with sidewalks, curbs, and roads. Urban Ecosyst. 2001, 5, 209–225. [Google Scholar] [CrossRef]
- Grabosky, J.; Bassuk, N.L. Seventeen years’ growth of street trees in structural soil compared with a tree lawn in New York City. Urban For. Urban Green. 2016, 16, 103–109. [Google Scholar] [CrossRef]
- Siegel-Issem, C.M.; Burger, J.A.; Powers, R.F.; Ponder, F.; Patterson, S.C. Seedling Root Growth as a Function of Soil Density and Water Content. Soil Sci. Soc. Am. J. 2005, 69, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Erena, S.H.; Worku, H. Dynamics of land use land cover and resulting surface runoff management for environmental flood hazard mitigation: The case of Dire Daw city, Ethiopia. J. Hydrol. Reg. Stud. 2019, 22, 100598. [Google Scholar] [CrossRef]
- Lesser, L.M. Hardscape damage by tree roots. J. Arboric. 2001, 27, 272–276. [Google Scholar]
- Gregory, J.H.; Dukes, M.D.; Jones, P.H.; Miller, G.L. Effect of urban soil compaction on infiltration rate. J. Soil Water Conserv. 2006, 61, 117–124. [Google Scholar]
- Frazer, L. Paving Paradise: The Peril of Impervious Surfaces. Environ. Health Perspect. 2005, 113, A456–A462. [Google Scholar] [CrossRef] [Green Version]
- Stratopoulos, L.M.F.; Zhang, C.; Häberle, K.-H.; Pauleit, S.; Duthweiler, S.; Pretzsch, H.; Rötzer, T. Effects of Drought on the Phenology, Growth, and Morphological Development of Three Urban Tree Species and Cultivars. Sustainability 2019, 11, 5117. [Google Scholar] [CrossRef] [Green Version]
- Tor-Ngern, P.; Panha, S. Species-specific Responses of Water Use by Urban Trees to Artificial Soil Drought: Results from a Small-scale Study. Appl. Environ. Res. 2016, 38, 53–60. [Google Scholar] [CrossRef]
- Nitschke, C.R.; Nichols, S.; Allen, K.; Dobbs, C.; Livesley, S.J.; Baker, P.; Lynch, Y. The influence of climate and drought on urban tree growth in southeast Australia and the implications for future growth under climate change. Landsc. Urban Plan. 2017, 167, 275–287. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.-K.; Su, Y.-B.; Zhang, H.-X. Land pavement depresses photosynthesis in urban trees especially under drought stress. Sci. Total Environ. 2019, 653, 120–130. [Google Scholar] [CrossRef]
- Shashua-Bar, L.; Pearlmutter, D.; Erell, E. The cooling efficiency of urban landscape strategies in a hot dry climate. Landsc. Urban Plan. 2009, 92, 179–186. [Google Scholar] [CrossRef]
- Takebayashi, H.; Moriyama, M. Study on Surface Heat Budget of Various Pavements for Urban Heat Island Mitigation. Adv. Mater. Sci. Eng. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Celestian, S.B.; Martin, C.A. Rhizosphere, surface, and air temperature patterns at parking lots in Phoenix, Arizona, U.S. J. Arboric. 2004, 30, 245–252. [Google Scholar]
- Doulos, L.T.; Santamouris, M.; Livada, I. Passive cooling of outdoor urban spaces. The role of materials. Sol. Energy 2004, 77, 231–249. [Google Scholar] [CrossRef]
- Lukac, M.; Calfapietra, C.; Lagomarsino, A.; Loreto, F. Global climate change and tree nutrition: Effects of elevated CO2 and temperature. Tree Physiol. 2010, 30, 1209–1220. [Google Scholar] [CrossRef] [Green Version]
- Dale, A.G.; Frank, S.D. Warming and drought combine to increase pest insect fitness on urban trees. PLoS ONE 2017, 12, e0173844. [Google Scholar] [CrossRef] [Green Version]
- Júnior, R.D.S.N.; do Amaral, G.C.; Pezzopane, J.E.M.; Toledo, J.V.; Xavier, T.M.T. Ecophysiology of C3 and C4 plants in terms of responses to extreme soil temperatures. Theor. Exp. Plant Physiol. 2018, 30, 261–274. [Google Scholar] [CrossRef]
- Lu, T.; Wang, K.-S. Numerical analysis of the heat transfer associated with freezing/solidifying phase changes for a pipeline filled with crude oil in soil saturated with water during pipeline shutdown in winter. J. Pet. Sci. Eng. 2008, 62, 52–58. [Google Scholar] [CrossRef]
- Yu, H.; Li, T.; Liu, Y.; Ma, L. Spatial distribution of polycyclic aromatic hydrocarbon contamination in urban soil of China. Chemosphere 2019, 230, 498–509. [Google Scholar] [CrossRef]
- Bernardino, C.A.R.; Mahler, C.F.; Santelli, R.E.; Freire, A.S.; Braz, B.F.; Novo, L.A.B. Metal accumulation in roadside soils of Rio de Janeiro, Brazil: Impact of traffic volume, road age, and urbanization level. Environ. Monit. Assess. 2019, 191, 156. [Google Scholar] [CrossRef]
- Suman, S.; Sinha, A.; Tarafdar, A. Polycyclic aromatic hydrocarbons (PAHs) concentration levels, pattern, source identification and soil toxicity assessment in urban traffic soil of Dhanbad, India. Sci. Total Environ. 2016, 545, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Kosheleva, N.E.; Nikiforova, E. Long-Term Dynamics of Urban Soil Pollution with Heavy Metals in Moscow. Appl. Environ. Soil Sci. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Fazeli, G.; Karbassi, A.R.; Khoramnejadian, S.; Nasrabadi, T. Evaluation of Urban Soil Pollution: A Combined Approach of Toxic Metals and Polycyclic Aromatic Hydrocarbons (PAHs). Int. J. Environ. Res. 2019, 13, 801–811. [Google Scholar] [CrossRef]
- Yaylalı-Abanuz, G. Application of multivariate statistics in the source identification of heavy-metal pollution in roadside soils of Bursa, Turkey. Arab. J. Geosci. 2019, 12, 382. [Google Scholar] [CrossRef]
- De Silva, S.; Ball, A.S.; Huynh, T.; Reichman, S. Metal accumulation in roadside soil in Melbourne, Australia: Effect of road age, traffic density and vehicular speed. Environ. Pollut. 2016, 208, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Cardelli, R.; Vanni, G.; Marchini, F.; Saviozzi, A. Characterization and origin of organic and inorganic pollution in urban soils in Pisa (Tuscany, Italy). Environ. Monit. Assess. 2017, 189, 554. [Google Scholar] [CrossRef]
- Wawer, M.; Magiera, T.; Ojha, G.; Appel, E.; Kusza, G.; Hu, S.; Basavaiah, N. Traffic-Related Pollutants in Roadside Soils of Different Countries in Europe and Asia. Water Air Soil Pollut. 2015, 226, 216. [Google Scholar] [CrossRef]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef]
- Azeez, J.O.; Mesele, S.; Sarumi, B.O.; Ogundele, J.A.; Uponi, A.O.; Hassan, A.O. Soil metal pollution as a function of traffic density and distance from road in emerging cities: A case study of Abeokuta, southwestern Nigeria. Arch. Agron. Soil Sci. 2014, 60, 275–295. [Google Scholar] [CrossRef]
- Sobrova, P.; Zehnalek, J.; Adam, V.; Beklova, M.; Kizek, R. The effects on soil/water/plant/animal systems by platinum group elements. Cent. Eur. J. Chem. 2012, 10, 1369–1382. [Google Scholar] [CrossRef]
- Li, J.; Jia, C.; Lu, Y.; Tang, S.; Shim, H. Multivariate analysis of heavy metal leaching from urban soils following simulated acid rain. Microchem. J. 2015, 122, 89–95. [Google Scholar] [CrossRef]
- Cunningham, M.A.; Snyder, E.; Yonkin, D.; Ross, M.; Elsen, T. Accumulation of deicing salts in soils in an urban environment. Urban Ecosyst. 2008, 11, 17–31. [Google Scholar] [CrossRef]
- Czerniawska-Kusza, I.; Kusza, G.; Dużyński, M. Effect of deicing salts on urban soils and health status of roadside trees in the Opole region. Environ. Toxicol. 2004, 19, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Migaszewski, Z.M.; Podlaski, R.; Dołęgowska, S.; Michalik, A. The influence of chloride deicers on mineral nutrition and the health status of roadside trees in the city of Kielce, Poland. Environ. Monit. Assess. 2011, 176, 451–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snieškienė, V.; Baležentienė, L.; Stankevičienė, A. Urban salt contamination impact on tree health and the prevalence of fungi agent in cities of the central Lithuania. Urban For. Urban Green. 2016, 19, 13–19. [Google Scholar] [CrossRef]
- Patykowski, J.; Kołodziejek, J.; Wala, M. Biochemical and growth responses of silver maple (Acer saccharinum L.) to sodium chloride and calcium chloride. PeerJ 2018, 6, e5958. [Google Scholar] [CrossRef] [Green Version]
- Equiza, M.; Calvo-Polanco, M.; Cirelli, D.; Señorans, J.; Wartenbe, M.; Saunders, C.; Zwiazek, J.J. Long-term impact of road salt (NaCl) on soil and urban trees in Edmonton, Canada. Urban For. Urban Green. 2017, 21, 16–28. [Google Scholar] [CrossRef]
- Newbound, M.; McCarthy, M.A.; Lebel, T. Fungi and the urban environment: A review. Landsc. Urban Plan. 2010, 96, 138–145. [Google Scholar] [CrossRef]
- Christoforidis, A.; Stamatis, N. Heavy metal contamination in street dust and roadside soil along the major national road in Kavala’s region, Greece. Geoderma 2009, 151, 257–263. [Google Scholar] [CrossRef]
- Omores, R.A.; Wewers, F.; Ikhide, P.O.; Farrar, T.; Giwa, A.-R. Spatio–Temporal Distribution of Polycyclic Aromatic Hydrocarbons in Urban Soils in Cape Town, South Africa. Int. J. Environ. Res. 2017, 11, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Pohrt, R. Tire Wear Particle Hot Spots—Review of Influencing Factors. Facta Univ. Ser. Mech. Eng. 2019, 17, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Kreider, M.L.; Unice, K.M.; Panko, J.M. Human health risk assessment of Tire and Road Wear Particles (TRWP) in air. Hum. Ecol. Risk Assess. Int. J. 2019, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, D.; Goel, A.; Agrawal, M. Particle Bound Metals at Major Intersections in an Urban Location and Source Identification through Use of Metal Markers. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2016, 86, 209–220. [Google Scholar] [CrossRef]
- Klöckner, P.; Reemtsma, T.; Eisentraut, P.; Braun, U.; Ruhl, A.S.; Wagner, S. Tire and road wear particles in road environment—Quantification and assessment of particle dynamics by Zn determination after density separation. Chemosphere 2019, 222, 714–721. [Google Scholar] [CrossRef]
- Földi, C.; Sauermann, S.; Dohrmann, R.; Mansfeldt, T. Traffic-related distribution of antimony in roadside soils. Environ. Pollut. 2018, 237, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, K.; Wells, M.; Kershaw, T. Utilising green and bluespace to mitigate urban heat island intensity. Sci. Total. Environ. 2017, 584, 1040–1055. [Google Scholar] [CrossRef]
- Hamada, S.; Ohta, T. Seasonal variations in the cooling effect of urban green areas on surrounding urban areas. Urban For. Urban Green. 2010, 9, 15–24. [Google Scholar] [CrossRef]
- Ponraj, M.; Lee, Y.Y.; Din, M.F.M.; Noor, Z.Z.; Iwao, K.; Chelliapan, S. Overview of Urban Heat Island (Uhi) Phenomenon towards Human Thermal Comfort. Environ. Eng. Manag. J. 2017, 16, 2097–2111. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, Y.; Wang, X.; Jia, T.; Xia, G.-S.; Ma, A.; Zhang, L. Pipeline leakage detection for district heating systems using multisource data in mid- and high-latitude regions. ISPRS J. Photogramm. Remote Sens. 2019, 151, 207–222. [Google Scholar] [CrossRef]
- Teskey, R.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Steppe, K. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 2015, 38, 1699–1712. [Google Scholar] [CrossRef]
- Reich, P.B.; Sendall, K.M.; Stefanski, A.; Wei, X.; Rich, R.; Montgomery, R.A. Boreal and temperate trees show strong acclimation of respiration to warming. Nature 2016, 531, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Katja, H.; Irina, B.; Hiie, I.; Olav, K.; Tiit, P.; Bahtijor, R.; Mari, T.; Ülo, N. Temperature responses of dark respiration in relation to leaf sugar concentration. Physiol. Plant. 2012, 144, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ren, G.; Hou, W. Temporal–Spatial Patterns of Relative Humidity and the Urban Dryness Island Effect in Beijing City. J. Appl. Meteorol. Clim. 2017, 56, 2221–2237. [Google Scholar] [CrossRef]
- Du, J.; Wang, K.; Jiang, S.; Cui, B.; Wang, J.; Zhao, C.; Li, J. Urban Dry Island Effect Mitigated Urbanization Effect on Observed Warming in China. J. Clim. 2019, 32, 5705–5723. [Google Scholar] [CrossRef]
- Wang, X.; Gong, Y. The impact of an urban dry island on the summer heat wave and sultry weather in Beijing City. Chin. Sci. Bull. 2010, 55, 1657–1661. [Google Scholar] [CrossRef]
- Bauer, H.; Ache, P.; Lautner, S.; Fromm, J.; Hartung, W.; Al-Rasheid, K.A.; Sonnewald, S.; Sonnewald, U.; Kneitz, S.; Lachmann, N.; et al. The Stomatal Response to Reduced Relative Humidity Requires Guard Cell-Autonomous ABA Synthesis. Curr. Biol. 2013, 23, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ouyang, Z.; Chen, W.; Wang, X.; Zheng, H.; Ren, Y. Water, heat, and airborne pollutants effects on transpiration of urban trees. Environ. Pollut. 2011, 159, 2127–2137. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Wang, B.; Wang, X.; Li, J.; Wu, J.; Liu, L. Interactive effects of air pollutants and atmospheric moisture stress on aspen growth and photosynthesis along an urban-rural gradient. Environ. Pollut. 2020, 260, 114076. [Google Scholar] [CrossRef]
- Moser-Reischl, A.; Rahman, M.A.; Pretzsch, H.; Pauleit, S.; Rötzer, T. Inter-and intraannual growth patterns of urban small-leaved lime (Tilia cordata mill.) at two public squares with contrasting microclimatic conditions. Int. J. Biometeorol. 2017, 61, 1095–1107. [Google Scholar] [CrossRef]
- Thomsen, S.; Reisdorff, C.; Gröngröft, A.; Jensen, K.; Eschenbach, A. Responsiveness of mature oak trees (Quercus robur L.) to soil water dynamics and meteorological constraints in urban environments. Urban Ecosyst. 2020, 23, 173–186. [Google Scholar] [CrossRef]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, R.K.; Shukla, A.; Parida, M.; Pandey, G. Urban roadside monitoring and prediction of CO, NO2 and SO2 dispersion from on-road vehicles in megacity Delhi. Transp. Res. Part D Transp. Environ. 2016, 46, 157–165. [Google Scholar] [CrossRef]
- Rogula-Kozłowska, W. Size-segregated urban particulate matter: Mass closure, chemical composition, and primary and secondary matter content. Air Qual. Atmos. Health 2016, 9, 533–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Liu, J.; Ban-Weiss, G.A.; Zhang, J.; Huang, X.; Ouyang, B.; Popoola, O.; Tao, S. Effects of canyon geometry on the distribution of traffic-related air pollution in a large urban area: Implications of a multi-canyon air pollution dispersion model. Atmos. Environ. 2017, 165, 111–121. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Z.; Teng, M.; Wang, P.; Zhou, L. Accumulation of three different sizes of particulate matter on plant leaf surfaces: Effect on leaf traits. Arch. Biol. Sci. 2015, 67, 1257–1267. [Google Scholar] [CrossRef]
- Dudley, B. BP Statistical Review of World Energy, 67th ed.; BP Statistical Review: London, UK, 2018. [Google Scholar]
- Yang, J.; Wang, Z.-H.; Kaloush, K.E. Environmental impacts of reflective materials: Is high albedo a ‘silver bullet’ for mitigating urban heat island? Renew. Sustain. Energy Rev. 2015, 47, 830–843. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Ma, P.; Bai, T.-H.; Wang, X.-Q.; Ma, F.-W. Effects of light intensity on photosynthesis and photoprotective mechanisms in apple under progressive drought. J. Integr. Agric. 2015, 14, 1755–1766. [Google Scholar] [CrossRef] [Green Version]
- Takebayashi, H.; Kasahara, M.; Tanabe, S.; Kouyama, M. Analysis of Solar Radiation Shading Effects by Trees in the Open Space around Buildings. Sustainability 2017, 9, 1398. [Google Scholar] [CrossRef] [Green Version]
- Tan, P.Y.; Ismail, M.R.B. Building shade affects light environment and urban greenery in high-density residential estates in Singapore. Urban For. Urban Green. 2014, 13, 771–784. [Google Scholar] [CrossRef]
- Takagi, M.; Gyokusen, K. Light and atmospheric pollution affect photosynthesis of street trees in urban environments. Urban For. Urban Green. 2004, 2, 167–171. [Google Scholar] [CrossRef]
- Kjelgren, R.K.; Clark, J.R. Photosynthesis and leaf morphology ofLiquidambar styraciflua L. under variable urban radiant-energy conditions. Int. J. Biometeorol. 1992, 36, 165–171. [Google Scholar] [CrossRef]
- Gebert, L.; Coutts, A.; Tapper, N. The influence of urban canyon microclimate and contrasting photoperiod on the physiological response of street trees and the potential benefits of water sensitive urban design. Urban For. Urban Green. 2019, 40, 152–164. [Google Scholar] [CrossRef]
- Spitschan, M.; Aguirre, G.K.; Brainard, D.H.; Sweeney, A.M. Variation of outdoor illumination as a function of solar elevation and light pollution. Sci. Rep. 2016, 6, 26756. [Google Scholar] [CrossRef] [PubMed]
- Ffrench-Constant, R.H.; Somers-Yeates, R.; Bennie, J.; Economou, T.; Hodgson, D.; Spalding, A.; McGregor, P.K. Light pollution is associated with earlier tree budburst across the United Kingdom. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160813. [Google Scholar] [CrossRef]
- Brelsford, C.; Robson, T.M. Blue light advances bud burst in branches of three deciduous tree species under short-day conditions. Trees 2018, 32, 1157–1164. [Google Scholar] [CrossRef]
- Brelsford, C.; Nybakken, L.; Kotilainen, T.; Robson, T.M. The influence of spectral composition on spring and autumn phenology in trees. Tree Physiol. 2019, 39, 925–950. [Google Scholar] [CrossRef]
- Škvareninová, J.; Tuhárska, M.; Škvarenina, J.; Babálová, D.; Slobodníková, L.; Slobodník, B.; Středová, H.; Minďáš, J. Effects of light pollution on tree phenology in the urban environment. Morav. Geogr. Rep. 2017, 25, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Nilsson, O. Molecular regulation of phenology in trees—Because the seasons they are a-changin’. Curr. Opin. Plant Biol. 2016, 29, 73–79. [Google Scholar] [CrossRef]
- Kwak, M.J.; Je, S.M.; Cheng, H.C.; Seo, S.M.; Park, J.H.; Baek, S.G.; Khaine, I.; Lee, T.; Jang, J.; Li, Y.; et al. Night Light-Adaptation Strategies for Photosynthetic Apparatus in Yellow-Poplar (Liriodendron tulipifera L.) Exposed to Artificial Night Lighting. Forests 2018, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Singhal, R.K.; Kumar, M.; Bose, B. Eco-physiological Responses of Artificial Night Light Pollution in Plants. Russ. J. Plant Physiol. 2019, 66, 190–202. [Google Scholar] [CrossRef]
- Chen, C.; Xiao, Y.-G.; Li, X.; Ni, M. Light-Regulated Stomatal Aperture in Arabidopsis. Mol. Plant 2012, 5, 566–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, D.; Douthe, C.; Flexas, J. Differential coordination of stomatal conductance, mesophyll conductance, and leaf hydraulic conductance in response to changing light across species. Plant Cell Environ. 2018, 41, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.W.; Hewitt, A.M.; Custic, M.; Lo, M. The Management of Tree Root Systems in Urban and Suburban Settings: A Review of Soil Influence on Root Growth. Arboric. Urban For. 2014, 40, 193–217. [Google Scholar]
- North, E.; D’Amato, A.W.; Russell, M.B.; Johnson, G.R. The influence of sidewalk replacement on urban street tree growth. Urban For. Urban Green. 2017, 24, 116–124. [Google Scholar] [CrossRef]
- Vogt, J.M.; Fischer, B.C. A Protocol for Citizen Science Monitoring of Recently-Planted Urban Trees. In Urban Forests, Ecosystem Services and Management; Blum, J., Ed.; Apple Academic Press: Oakville, ON, Canada, 2014; pp. 153–186. [Google Scholar]
- Allen, K.; Harper, R.W.; Bayer, A.; Brazee, N.J. A review of nursery production systems and their influence on urban tree survival. Urban For. Urban Green. 2017, 21, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C. Air pollution removal by urban trees and shrubs in the United States. Urban For. Urban Green. 2006, 4, 115–123. [Google Scholar] [CrossRef]
- Timonen, S.; Kauppinen, P. Mycorrhizal colonisation patterns of Tilia trees in street, nursery and forest habitats in southern Finland. Urban For. Urban Green. 2008, 7, 265–276. [Google Scholar] [CrossRef]
- Fietz, M.; Burger, H. Strassenbaum-Zustandsbericht Berliner Innenstadt 2015; Ergebnisse der Straßenbaum-Zustandserhebung aus CIR-Luftbildern; Senatsverwaltung für Stadtentwicklung, I C: Berlin, Germany, 2016. (In German) [Google Scholar]
- Roman, L.A.; Scatena, F.N. Street tree survival rates: Meta-analysis of previous studies and application to a field survey in Philadelphia, PA, USA. Urban For. Urban Green. 2011, 10, 269–274. [Google Scholar] [CrossRef]
- Ko, Y.; Lee, J.-H.; McPherson, E.G.; Roman, L.A. Factors affecting long-term mortality of residential shade trees: Evidence from Sacramento, California. Urban For. Urban Green. 2015, 14, 500–507. [Google Scholar] [CrossRef]
- Sherman, A.R.; Kane, B.; Autio, W.A.; Harris, J.R.; Ryan, H.D.P. Establishment period of street trees growing in the Boston, MA metropolitan area. Urban For. Urban Green. 2016, 19, 95–102. [Google Scholar] [CrossRef]
- Roman, L.A.; Walker, L.A.; Martineau, C.M.; Muffly, D.J.; MacQueen, S.A.; Harris, W. Stewardship matters: Case studies in establishment success of urban trees. Urban For. Urban Green. 2015, 14, 1174–1182. [Google Scholar] [CrossRef] [Green Version]
- Vogt, J.M.; Watkins, S.L.; Mincey, S.K.; Patterson, M.S.; Fischer, B.C. Explaining planted-tree survival and growth in urban neighborhoods: A social–ecological approach to studying recently-planted trees in Indianapolis. Landsc. Urban Plan. 2015, 136, 130–143. [Google Scholar] [CrossRef] [Green Version]
- Roman, L.A.; Battles, J.J.; McBride, J.R. Urban Tree Mortality: A Primer on Demographic Approaches; General Technical Report NRS-158; US Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2016; Volume 158, pp. 1–24. [Google Scholar]
- Pauleit, S.; Jones, N.; Garcia-Martin, G.; Garcia-Valdecantos, J.L.; Rivière, L.M.; Vidal-Beaudet, L.; Bodson, M.; Randrup, T.B. Tree establishment practice in towns and cities—Results from a European survey. Urban For. Urban Green. 2002, 1, 83–96. [Google Scholar] [CrossRef]
- Hamzah, H.; Othman, N.; Hussain, N.H.M.; Simis, M. The criteria of urban trees regarding the issues of tree vandalism. IOP Conf. Ser. Earth Environ. Sci. 2018, 203, 012023. [Google Scholar] [CrossRef]
- Badrulhisham, N.; Othman, N. Knowledge in Tree Pruning for Sustainable Practices in Urban Setting: Improving Our Quality of Life. Procedia Soc. Behav. Sci. 2017, 234, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Dmuchowski, W.; Baczewska, A.; Brągoszewska, P. Reaction of street trees to adverse environmental conditions in the centre of Warsaw. Ecol. Quest. 2011, 15, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Forrest, M.; Konijnendijk, C. A History of Urban Forests and Trees in Europe. In Urban Forests and Trees; Konijnendijk, C., Nilsson, K., Randrup, T., Schipperijn, J., Eds.; Springer: Berlin, Germany, 2005; pp. 23–48. [Google Scholar]
- Seamans, G.S. Mainstreaming the environmental benefits of street trees. Urban For. Urban Green. 2013, 12, 2–11. [Google Scholar] [CrossRef]
- Tzoulas, K.; Korpela, K.; Venn, S.; Yli-Pelkonen, V.; Kaźmierczak, A.; Niemelä, J.; James, P. Promoting ecosystem and human health in urban areas using Green Infrastructure: A literature review. Landsc. Urban Plan. 2007, 81, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Donovan, G.H. Including public-health benefits of trees in urban-forestry decision making. Urban For. Urban Green. 2017, 22, 120–123. [Google Scholar] [CrossRef]
- Brady, E. Aesthetic Value, Nature, and Environment. In The Oxford Handbook of Environmental Ethics; Gardiner, S.M., Thompson, A., Eds.; Oxford University Press: New York, NY, USA, 2017; pp. 186–198. [Google Scholar]
- O’Brien, L.; De Vreese, R.; Kern, M.; Sievänen, T.; Stojanova, B.; Atmiş, E. Cultural ecosystem benefits of urban and peri-urban green infrastructure across different European countries. Urban For. Urban Green. 2017, 24, 236–248. [Google Scholar] [CrossRef]
- Watts, G. The effects of “greening” urban areas on the perceptions of tranquillity. Urban For. Urban Green. 2017, 26, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Donovan, G.H.; Prestemon, J.P. The Effect of Trees on Crime in Portland, Oregon. Environ. Behav. 2012, 44, 3–30. [Google Scholar] [CrossRef]
- Kuo, F.E.; Sullivan, W.C. Environment and Crime in the Inner City. Does Vegetation Reduce Crime? Environ. Behav. 2001, 33, 343–367. [Google Scholar] [CrossRef]
- Locke, D.H.; Han, S.; Kondo, M.C.; Murphy-Dunning, C.; Cox, M. Did community greening reduce crime? Evidence from New Haven, CT, 1996–2007. Landsc. Urban Plan. 2017, 161, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Escobedo, F.J.; Clerici, N.; Staudhammer, C.L.; Feged-Rivadeneira, A.; Bohorquez, J.C.; Tovar, G. Trees and Crime in Bogota, Colombia: Is the link an ecosystem disservice or service? Land Use Policy 2018, 78, 583–592. [Google Scholar] [CrossRef]
- Tarran, J. People and trees, providing benefits, overcoming impediments. In Proceedings of the 10th National Street Tree Symposium 2009, Adelaide, South Australia, 3 September 2009; Lawry, D., Gardner, J., Bridget, M., Eds.; The University of Adelaide Australia: Adelaide, Australia, 2009; pp. 63–82. [Google Scholar]
- Thompson, C.W.; Roe, J.J.; Aspinall, P.A.; Mitchell, R.; Clow, A.; Miller, D. More green space is linked to less stress in deprived communities: Evidence from salivary cortisol patterns. Landsc. Urban Plan. 2012, 105, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Li, D.; Larsen, L.; Sullivan, W.C. A Dose-Response Curve Describing the Relationship between Urban Tree Cover Density and Self-Reported Stress Recovery. Environ. Behav. 2016, 48, 607–629. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, M.; Jane, H.-A.; Li, S.; Bauer, N. Trees, grass, or concrete? The effects of different types of environments on stress reduction. Landsc. Urban Plan. 2020, 193, 103654. [Google Scholar] [CrossRef]
- Ulmer, J.M.; Wolf, K.L.; Backman, D.R.; Tretheway, R.L.; Blain, C.J.; O’Neil-Dunne, J.P.; Frank, L.D. Multiple health benefits of urban tree canopy: The mounting evidence for a green prescription. Health Place 2016, 42, 54–62. [Google Scholar] [CrossRef]
- Van Dillen, S.M.E.; De Vries, S.; Groenewegen, P.P.; Spreeuwenberg, P. Greenspace in urban neighbourhoods and residents’ health: Adding quality to quantity. J. Epidemiol. Community Health 2012, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandit, R.; Polyakov, M.; Sadler, R. The importance of tree cover and neighbourhood parks in determining urban property values. In Proceedings of the 56th AARES Annual Conference, The Importance of Tree Cover and Neighbourhood Parks in Determining Urban Property Values, Fremantle, Australia, 7–10 February 2012; pp. 1–16. [Google Scholar]
- Pandit, R.; Polyakov, M.; Tapsuwan, S.; Moran, T. The effect of street trees on property value in Perth, Western Australia. Landsc. Urban Plan. 2013, 110, 134–142. [Google Scholar] [CrossRef]
- Setälä, H.M.; Francini, G.; Allen, J.; Jumpponen, A.; Hui, N.; Kotze, D.J. Urban parks provide ecosystem services by retaining metals and nutrients in soils. Environ. Pollut. 2017, 231, 451–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasevic, M.; Rajšić, S.; Đorđević, D.; Tasić, M.; Krstić, J.B.; Novaković, V.T. Heavy metals accumulation in tree leaves from urban areas. Environ. Chem. Lett. 2004, 2, 151–154. [Google Scholar] [CrossRef]
- Unterbrunner, R.; Puschenreiter, M.; Sommer, P.; Wieshammer, G.; Tlustoš, P.; Zupan, M.; Wenzel, W. Heavy metal accumulation in trees growing on contaminated sites in Central Europe. Environ. Pollut. 2007, 148, 107–114. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Cheng, W. Interactions between soil and tree roots accelerate long-term soil carbon decomposition. Ecol. Lett. 2007, 10, 1046–1053. [Google Scholar] [CrossRef]
- Reubens, B.; Poesen, J.; Danjon, F.; Geudens, G.; Muys, B. The role of fine and coarse roots in shallow slope stability and soil erosion control with a focus on root system architecture: A review. Trees 2007, 21, 385–402. [Google Scholar] [CrossRef]
- Armson, D.; Stringer, P.; Ennos, A. The effect of street trees and amenity grass on urban surface water runoff in Manchester, UK. Urban For. Urban Green. 2013, 12, 282–286. [Google Scholar] [CrossRef]
- Berland, A.; Shiflett, S.A.; Shuster, W.D.; Garmestani, A.S.; Goddard, H.; Herrmann, D.L.; Hopton, M.E. The role of trees in urban stormwater management. Landsc. Urban Plan. 2017, 162, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Peper, P.J.; McPherson, E.G.; Simpson, J.R.; Gardner, S.L.; Vargas, K.E.; Xiao, Q. New York City, New York Municipal Forest Resource Analysis; Center for Urban Forest Research, United States Department of Agriculture, Forest Service, Pacific Southwest Research Station: Washington, DC, USA, 2007; pp. 1–65. [Google Scholar]
- Lindén, J.; Fonti, M.V.; Esper, J. Temporal variations in microclimate cooling induced by urban trees in Mainz, Germany. Urban For. Urban Green. 2016, 20, 198–209. [Google Scholar] [CrossRef]
- Coutts, A.M.; White, E.C.; Tapper, N.J.; Beringer, J.; Livesley, S.J. Temperature and human thermal comfort effects of street trees across three contrasting street canyon environments. Theor. Appl. Climatol. 2015, 124, 55–68. [Google Scholar] [CrossRef]
- Speak, A.; Montagnani, L.; Wellstein, C.; Zerbe, S. The influence of tree traits on urban ground surface shade cooling. Landsc. Urban Plan. 2020, 197, 103748. [Google Scholar] [CrossRef]
- Kántor, N.; Chen, L.; Gál, C.V. Human-biometeorological significance of shading in urban public spaces—Summertime measurements in Pécs, Hungary. Landsc. Urban Plan. 2018, 170, 241–255. [Google Scholar] [CrossRef]
- Kong, L.; Lau, K.K.L.; Yuan, C.; Chen, Y.; Xu, Y.; Ren, C.; Ng, E. Regulation of outdoor thermal comfort by trees in Hong Kong. Sustain. Cities Soc. 2017, 31, 12–25. [Google Scholar] [CrossRef]
- Konarska, J.; Uddling, J.; Holmer, B.; Lutz, M.; Lindberg, F.; Pleijel, H.; Thorsson, S. Transpiration of urban trees and its cooling effect in a high latitude city. Int. J. Biometeorol. 2016, 60, 159–172. [Google Scholar] [CrossRef]
- Scholz, T.; Hof, A.; Schmitt, T. Cooling Effects and Regulating Ecosystem Services Provided by Urban Trees—Novel Analysis Approaches Using Urban Tree Cadastre Data. Sustainability 2018, 10, 712. [Google Scholar] [CrossRef] [Green Version]
- Gillner, S.; Vogt, J.; Tharang, A.; Dettmann, S.; Roloff, A. Role of street trees in mitigating effects of heat and drought at highly sealed urban sites. Landsc. Urban Plan. 2015, 143, 33–42. [Google Scholar] [CrossRef]
- Rahman, M.A.; Armson, D.; Ennos, R. A comparison of the growth and cooling effectiveness of five commonly planted urban tree species. Urban Ecosyst. 2015, 18, 371–389. [Google Scholar] [CrossRef]
- Chen, B.; Li, S.; Yang, X.; Lu, S.; Wang, B.; Niu, X. Characteristics of atmospheric PM2.5 in stands and non-forest cover sites across urban-rural areas in Beijing, China. Urban Ecosyst. 2016, 19, 867–883. [Google Scholar] [CrossRef]
- Sæbø, A.; Hanslin, H.M.; Torp, T.; Lierhagen, S.; Gawrońska, H.; Dzierzanowski, K.; Gawronski, S.W. Chemical composition of vegetation along urbanisation gradients in two European cities. Environ. Pollut. 2015, 198, 116–125. [Google Scholar] [CrossRef]
- Dzierżanowski, K.; Popek, R.; Gawrońska, H.; Sæbø, A.; Gawronski, S.W. Deposition of Particulate Matter of Different Size Fractions on Leaf Surfaces and in Waxes of Urban Forest Species. Int. J. Phytoremediat. 2011, 13, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chang, Y.; Yan, P. Ranking the suitability of common urban tree species for controlling PM2.5 pollution. Atmos. Pollut. Res. 2015, 6, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Shi, H.; Li, Y.; Yu, Y.; Zhang, J. Seasonal variations in leaf capturing of particulate matter, surface wettability and micromorphology in urban tree species. Front. Environ. Sci. Eng. 2013, 7, 579–588. [Google Scholar] [CrossRef]
- Chen, L.; Liu, C.; Zhang, L.; Zou, R.; Zhang, Z. Variation in Tree Species Ability to Capture and Retain Airborne Fine Particulate Matter (PM2.5). Sci. Rep. 2017, 7, 3206. [Google Scholar] [CrossRef] [PubMed]
- Gratani, L.; Varone, L.; Bonito, A. Carbon sequestration of four urban parks in Rome. Urban For. Urban Green. 2016, 19, 184–193. [Google Scholar] [CrossRef]
- De Nicola, F.; Maisto, G.; Prati, M.V.; Alfani, A. Leaf accumulation of trace elements and polycyclic aromatic hydrocarbons (PAHs) in Quercus ilex L. Environ. Pollut. 2008, 153, 376–383. [Google Scholar] [CrossRef]
- Selmi, W.; Weber, C.; Rivière, E.; Blond, N.; Mehdi, L.; Nowak, D. Air pollution removal by trees in public green spaces in Strasbourg city, France. Urban For. Urban Green. 2016, 17, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Nawrot, B.; Dzierżanowski, K.; Gawroński, S.W. Accumulation of Particulate Matter, PAHs and Heavy Metals in Canopy of Small-Leaved Lime; Environmental Protection and Natural Resources: Warsaw, Poland, 2011; Volume 49, pp. 52–60. [Google Scholar]
- Jose, S. Agroforestry for ecosystem services and environmental benefits: An overview. Agrofor. Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Bottalico, F.; Chirici, G.; Giannetti, F.; De Marco, A.; Nocentini, S.; Paoletti, E.; Salbitano, F.; Sanesi, G.; Serenelli, C.; Travaglini, D. Air Pollution Removal by Green Infrastructures and Urban Forests in the City of Florence. Agric. Agric. Sci. Procedia 2016, 8, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C.; Ibarra, M. Brooklyn’s Urban Forest; General Technical Report NE-290; Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Newtown Square, PA, USA, 2002. [Google Scholar]
- Farina, A. Human Dimension of the Soundscape: From Individuals to Society. In Soundscape Ecology. Principles, Patterns, Methods and Applications; Springer: Dordrecht, The Netherlands, 2014; pp. 107–142. [Google Scholar] [CrossRef]
- Van Renterghem, T. Towards explaining the positive effect of vegetation on the perception of environmental noise. Urban For. Urban Green. 2019, 40, 133–144. [Google Scholar] [CrossRef]
- Mullaney, J.; Lucke, T.; Trueman, S.J. A review of benefits and challenges in growing street trees in paved urban environments. Landsc. Urban Plan. 2015, 134, 157–166. [Google Scholar] [CrossRef]
- Millward, A.A.; Sabir, S. Benefits of a forested urban park: What is the value of Allan Gardens to the city of Toronto, Canada? Landsc. Urban Plan. 2011, 100, 177–188. [Google Scholar] [CrossRef]
- Akbari, H.; Pomerantz, M.; Taha, H. Cool surfaces and shade trees to reduce energy use and improve air quality in urban areas. Sol. Energy 2001, 70, 295–310. [Google Scholar] [CrossRef]
- Soares, A.; Rego, F.C.; McPherson, E.; Simpson, J.; Peper, P.; Xiao, Q. Benefits and costs of street trees in Lisbon, Portugal. Urban For. Urban Green. 2011, 10, 69–78. [Google Scholar] [CrossRef]
- Zhang, Y.; Hussain, A.; Deng, J.; Letson, N. Public Attitudes Toward Urban Trees and Supporting Urban Tree Programs. Environ. Behav. 2007, 39, 797–814. [Google Scholar] [CrossRef]
- McPherson, E.G. Selecting reference cities for i-Tree Streets. Arboric. Urban For. 2010, 36, 230–240. [Google Scholar]
- Cariñanos, P.; Calaza-Martínez, P.; O’Brien, L.; Calfapietra, C. The cost of greening: Disservices of urban trees. In The Urban Forest, Cultivating Green Infrastructure for People and the Environment; Pearlmutter, D., Calfapietra, C., Samson, R., O’Brien, L., Krajter Ostoić, S., Sanesi, G., Del Amo, R.A., Eds.; Springer: Cham, Switzerland, 2017; pp. 79–87. [Google Scholar] [CrossRef]
- Weissteiner, C.; Rauch, H.P. Field data analysis of asphalt road paving damages caused by tree roots. Geophys. Res. Abstr. 2015, 17, 1. [Google Scholar]
- Obradović, D. The impact of tree root systems on wastewater pipes. In Proceedings of the Peti Skup Mladih Istraživača iz Područja Građevinarstva i Srodnih Tehničkih Znanosti Zajednički Temelji, Zagreb, Croatia, 18–19 September 2017; Volume 17, pp. 65–71. [Google Scholar]
- Li, J.; Guo, L. Field Investigation and Numerical Analysis of Residential Building Damaged by Expansive Soil Movement Caused by Tree Root Drying. J. Perform. Constr. Facil. 2017, 31, D4016003. [Google Scholar] [CrossRef]
- Palmer, M.A.; Liu, J.; Matthews, J.H.; Mumba, M.; D’Odorico, P. Water security: Gray or green? In: Manage water in a green way. American Association for the Advancement of Science, 2015. Science 2015, 349, 584. [Google Scholar] [CrossRef] [Green Version]
- Nicoll, B.; Armstrong, A. Development of Prunus Root Systems in A City Street: Pavement Damage and Root Architecture. Arboric. J. 1998, 22, 259–270. [Google Scholar] [CrossRef]
- Koeser, A.; Hauer, R.; Norris, K.; Krouse, R. Factors influencing long-term street tree survival in Milwaukee, WI, USA. Urban For. Urban Green. 2013, 12, 562–568. [Google Scholar] [CrossRef]
- Khedive, E.; Shirvany, A.; Assareh, M.H.; Sharkey, T.D. In situ emission of BVOCs by three urban woody species. Urban For. Urban Green. 2017, 21, 153–157. [Google Scholar] [CrossRef]
- McCormick, A.C.; Irmisch, S.; Boeckler, G.A.; Gershenzon, J.; Köllner, T.G.; Unsicker, S.B. Herbivore-induced volatile emission from old-growth black poplar trees under field conditions. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, A.; Kumar, P. Impact evaluation of green–grey infrastructure interaction on built-space integrity: An emerging perspective to urban ecosystem service. Sci. Total Environ. 2014, 487, 350–360. [Google Scholar] [CrossRef] [Green Version]
- Mrđan, S.; Ljubojević, M.; Orlović, S.; Čukanović, J.; Dulić, J. Poisonous and allergenic plant species in preschool’s and primary school’s yards in the city of Novi Sad. Urban For. Urban Green. 2017, 25, 112–119. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czaja, M.; Kołton, A.; Muras, P. The Complex Issue of Urban Trees—Stress Factor Accumulation and Ecological Service Possibilities. Forests 2020, 11, 932. https://0-doi-org.brum.beds.ac.uk/10.3390/f11090932
Czaja M, Kołton A, Muras P. The Complex Issue of Urban Trees—Stress Factor Accumulation and Ecological Service Possibilities. Forests. 2020; 11(9):932. https://0-doi-org.brum.beds.ac.uk/10.3390/f11090932
Chicago/Turabian StyleCzaja, Monika, Anna Kołton, and Piotr Muras. 2020. "The Complex Issue of Urban Trees—Stress Factor Accumulation and Ecological Service Possibilities" Forests 11, no. 9: 932. https://0-doi-org.brum.beds.ac.uk/10.3390/f11090932