Anthropogenic Factors Control the Distribution of a Southern Conifer Phytophthora Disease in a Peri-Urban Area of Northern Patagonia, Argentina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species and Study Area
2.2. Austrocedrus Disease
2.3. Anthropic and Environmental Properties
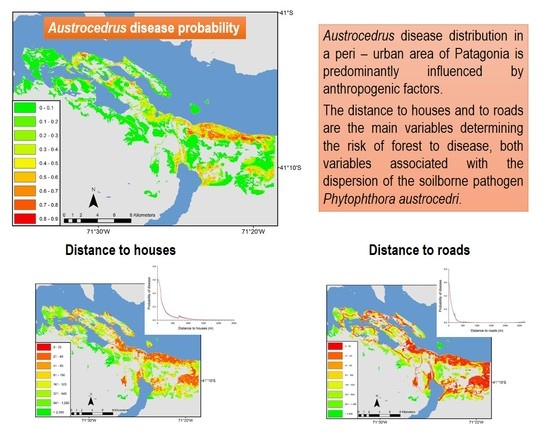
2.4. Risk Model—Landscape Scale Study
2.5. Local Scale Study
3. Results
3.1. Landscape Scale Study
3.2. Local Scale Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manion, P.D. Tree Disease Concepts, 2nd ed.; Prentice-Hall: Englewood Cliffs, NY, USA, 1991. [Google Scholar]
- Ciesla, W.M.; Donaubauer, E. Decline and Dieback of Trees and Forests. A Global Overview; FAO: Rome, Italy, 1994; p. 90. [Google Scholar]
- Waring, K.M.; O′Hara, K.L. Silvicultural strategies in forest ecosystems affected by introduced pests. For. Ecol. Manag. 2005, 209, 27–41. [Google Scholar] [CrossRef]
- Del Campo, A.D.; Fernandes, T.J.; Molina, A.J. Hydrology-oriented (adaptive) silviculture in a semiarid pine plantation: How much can be modified the water cycle through forest management? Eur. J. For. Res. 2014, 133, 879–894. [Google Scholar] [CrossRef]
- Recanatesi, F.; Giuliani, C.; Ripa, M.N. Monitoring Mediterranean Oak Decline in a Peri-Urban Protected Area Using the NDVI and Sentinel-2 Images: The Case Study of Castelporziano State Natural Reserve. Sustainability 2018, 10, 3308. [Google Scholar] [CrossRef] [Green Version]
- Romagnoli, M.; Moroni, S.; Recanatesi, F.; Salvati, R.; Scarascia Mugnozza, G. Climate factors and oak decline based on tree-ring analysis. A case study of peri-urban forest in the Mediterranean area. Urban For. Urban Green. 2018, 34, 17–28. [Google Scholar] [CrossRef]
- La Manna, L.; Rajchenberg, M. The decline of Austrocedrus chilensis forests in Patagonia, Argentina: Soil features as predisposing factors. For. Ecol. Manag. 2004, 190, 345–357. [Google Scholar] [CrossRef]
- La Manna, L.; Rajchenberg, M. Soil properties and Austrocedrus chilensis decline in Central Patagonia, Argentina. Plant Soil. 2004, 263, 29–41. [Google Scholar] [CrossRef]
- Greslebin, A.G.; Hansen, E.M. Pathogenicity of Phytophthora austrocedrae on Austrocedrus chilensis and its relation with ‘‘Mal del Ciprés’’ in Patagonia. Plant Pathol. 2010, 59, 604–612. [Google Scholar] [CrossRef]
- Vélez, M.L.; Coetzee, M.P.A.; Wingfield, M.J.; Rajchenberg, M.; Greslebin, A.G. Evidence of low levels of genetic diversity for the Phytophthora austrocedrae population in Patagonia, Argentina. Plant Pathol. 2014, 63, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Taccari, L.E.; Greslebin, A.G.; Salgado Salomón, M.E.; Vélez, M.L. Two conifer species native to Patagonia threatened by Phytophthora austrocedri. For. Pathol. 2019, 49, e12496. [Google Scholar] [CrossRef]
- Garbelotto, M.; Hayden, K.J. Sudden Oak Death: Interactions of the Exotic Oomycete Phytophthora ramorum with Naïve North American Hosts. Am. Soc. Microbiol. 2012, 11, 1313–1323. [Google Scholar] [CrossRef] [Green Version]
- El Mujtar, V. Análisis Integrado de Factores Genéticos, Bióticos y Abióticos Para la Formulación de Una Nueva Hipótesis Sobre la Etiología del “mal del Ciprés”. Ph.D. Thesis, Universidad Nacional de la Plata, La Plata, Argentina, 2009. [Google Scholar]
- Havrylenko, M.; Rosso, P.; Fontenla, S. Austrocedrus chilensis. Contribución al estudio de la mortalidad en Argentina. Bosque 1989, 10, 29–36. [Google Scholar] [CrossRef]
- Vélez, M.L.; Silva, P.V.; Troncoso, O.A.; Greslebin, A.G. Alteration of physiological parameters of Austrocedrus chilensis by the pathogen Phytophthora austrocedrae. Plant Pathol. 2012, 61, 877–888. [Google Scholar] [CrossRef]
- Troncoso, O.; Greslebin, A. Trabeculae in Patagonian mountain cypress (Austrocedrus chilensis) associated with Phytophthora austrocedri infection. IAWA J. 2018, 39, 209–220. [Google Scholar] [CrossRef]
- Barroetaveña, C.; Rajchenberg, M. Hongos Aphyllophorales (Basidiomycetes) que causan pudriciones en Austrocedrus chilensis en pie. Bol. Soc. Arg. Bot. 1996, 31, 201–216. [Google Scholar]
- Kitzberger, T. Ecotones as complex arenas of disturbance, climate, and human Impacts: The trans-Andean forest-steppe ecotone of Northern Patagonia. In Ecotones between Forest and Grassland; Myster, R., Ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Dondo Bühler, M.; de Torres Curth, M.; Garibaldi, L.A. Demography and socioeconomic vulnerability influence fire occurrence in Bariloche (Argentina). Landsc. Urb. Plann. 2013, 110, 64–73. [Google Scholar] [CrossRef]
- Plaza, P.I.; Speziale, K.L.; Zamora-Nasca, L.B.; Lambertucci, S.A. Dogs and cats put wildlife at risk. J. Wildl. Manage. 2019, 83, 767–768. [Google Scholar] [CrossRef]
- Ball, D.J.; Watt, J. The risk to the public of tree fall. J. Risk Res. 2013, 261, 1366–9877. [Google Scholar] [CrossRef]
- Bahamonde, H.; Peri, P.L.; Gargaglione, V.; Díaz, B.; Monelos, L.; Almonacid, L. Arbolado urbano en Patagonia sur. Principales Especies y su Manejo; Consejo Agrario Provincial: Río Gallegos, Argentina, 2018; p. 169. [Google Scholar]
- Bacalá, N.B.; Rosso, P.H.; Havrylenko, M. Austrocedrus chilensis mortality in the Nahuel Huapí Park (Argentina). For. Ecol. Manag. 1998, 109, 261–269. [Google Scholar] [CrossRef]
- La Manna, L.; Mateucci, S.D.; Kitzberger, T. Abiotic factors related to the incidence of Austrocedrus chilensis disease at a landscape scale. For. Ecol. Manag. 2008, 256, 1087–1095. [Google Scholar] [CrossRef]
- La Manna, L.; Matteucci, S.D.; Kitzberger, T. Modelling potential Phytophthora disease risk in Austrocedrus chilensis forests of Patagonia. Eur. J. For. Res. 2012, 131, 323–337. [Google Scholar] [CrossRef]
- La Manna, L.; Greslebin, A.; Matteucci, S.D. Avance de la mortalidad de los bosques de Austrocedrus chilensis a escala de paisaje. Rev. Asoc. Arg. Ecol. Paisaje 2014, 5, 17–24. [Google Scholar]
- Erwin, D.; Ribeiro, O. Phytophthora Diseases Worldwide; APS Press: St Paul, MN, USA, 1996; p. 562. [Google Scholar]
- Greslebin, A.G.; Hansen, E.M.; Sutton, W. Phytophthora austrocedrae sp. nov., a new species associated with Austrocedrus chilensis mortality in Patagonia (Argentina). Mycol. Res. 2007, 111, 308–316. [Google Scholar] [CrossRef]
- Donald, F.; Green, S.; Searle, K.; Cunniffe, N.J.; Purse, B.V. Small scale variability in soil moisture drives infection of vulnerable juniper populations by invasive forest pathogen. For. Ecol. Manag. 2020, 473, 118324. [Google Scholar] [CrossRef]
- Hansen, E.; Goheen, D.; Jules, E.; Ullian, B. Managing Port-Orford-Cedar and the Introduced Pathogen Phytophthora lateralis. Plant Dis. 1999, 84, 4–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meentemeyer, R.; Rizzo, D.; Mark, W.; Lotz, E. Mapping the risk of establishment and spread of sudden oak death in California. For. Ecol. Manag. 2004, 200, 195–214. [Google Scholar] [CrossRef]
- Venette, R.C.; Cohen, S.D. Potential climatic suitability for establishment of Phytophthora ramorum within the contiguous United States. For. Ecol. Manag. 2006, 231, 18–26. [Google Scholar] [CrossRef]
- Phillips, S.; Dudík, M. Modeling of species distribution wit Maxent: New extensions and comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Phillips, S.; Anderson, R.; Schapired, R. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Pearson, R.; Raxworthy, C.; Nakamura, M.; Townsend, P. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Dimitri, M. La Región de los Bosques Andino-Patagónicos II. Flora Dendrológica y Cultivada; Colección Científica del INTA: Buenos Aires, Argentina, 1982. [Google Scholar]
- Dezzoti, A.; Sancholuz, L. Los bosques de Austrocedrus chilensis en Argentina: Ubicación, estructura y crecimiento. Bosque 1991, 12, 43–52. [Google Scholar] [CrossRef]
- La Manna, L. Caracterización de los suelos bajo bosque de Austrocedrus chilensis a través de un gradiente climático y topográfico en Chubut, Argentina. Bosque 2005, 26, 137–153. [Google Scholar] [CrossRef]
- Núñez, C.; Pérez, A.; Raponi, C. Maps of Austrocedrus chilensis forests affected by dieback. In Proceedings of the 7th Meeting of the International Union of Forest Research Organizations, IUFRO Working Party 7.02.09, Phytophthora in Forests & Natural Ecosystems, Chubut, Argentina, 10–14 November 2014; p. 169. [Google Scholar]
- Andean Forest Service. La problemática Sanitaria del Ciprés de la Cordillera en los Ecosistemas Urbanos de San Carlos de Bariloche; Secretaría de Producción, Dirección de Bosques, Servicio Forestal Andino: San Carlos de Bariloche, Argentina, 2012; p. 15. [Google Scholar]
- Baldini, A.; Oltremari, J.; Holmgren, A. Efecto de Cinara cupressi (Hemiptera: Aphididae) sobre el ciprés de la cordillera (Austrocedrus chilensis) después de aplicar control químico. Cienc. Investig. Agrar. 2008, 35, 341–350. [Google Scholar] [CrossRef]
- Peña, M.A.; Altmann, S.H. Reconocimiento del efecto de Cinara cupressi (Hemiptera: Aphididae) en el estado sanitario de Austrocedrus chilensis mediante imágenes multiespectrales. Bosque 2009, 30, 151–158. [Google Scholar] [CrossRef]
- Barros, V.; Cordon, V.; Moyano, C.; Mendez, R.; Forquera, J.; Pizzio, O. Cartas de Precipitación de la Zona Oeste de las Provincias de Río Negro y Neuquén; Universidad Nacional del Comahue: Neuquén, Argentina, 1983; p. 66. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2018. Available online: http://qgis.osgeo.org (accessed on 21 September 2019).
- Anchorena, J.; Cingolani, A. Identifying habitat types in a disturbed area of the forest-steppe ecotone of Patagonia. Plant Ecol. 2002, 158, 97–112. [Google Scholar] [CrossRef]
- Servicio Forestal Andino de la Provincia de Río Negro. Mapa de Vegetación de la Provincia de Rio Negro; Consejo de Ecología y Medioambiente de la Provincia de Río Negro: San Carlos de Bariloche, Argentina, 2010. [Google Scholar]
- Manel, S.; Williams, H.C.; Ormerod, S.J. Evaluating presences absence models in ecology: The need to account for prevalence. J. Appl. Ecol. 2001, 38, 921–931. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.; Dawson, T.; Pearson, R. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [Green Version]
- La Manna, L.; Greslebin, A.; Matteucci, S.D. Applying Cost-distance analysis for forest disease risk mapping: Phytophthora austrocedrae as an example. Eur. J. For. Res. 2013, 132, 877–885. [Google Scholar] [CrossRef]
- Cushman, J.H.; Meentemeyer, R.K. Multi-scale patterns of human activity and the incidence of an exotic forest pathogen. J. Ecol. 2008, 96, 766–776. [Google Scholar] [CrossRef]
- Redondo, M.; Boerg, J.; Stenlid, J.; Oliva, J. Functional traits associated with the establishment of introduced Phytophthora spp. in Swedish forests. J. Appl. Ecol. 2018, 55, 1538–1552. [Google Scholar] [CrossRef]
- CABI. Phytophthora austrocedri [original text by Green, S.]. In Invasive Species Compendium; CAB International: Wallingford, UK, 2020. [Google Scholar]
- Colmet Dâage, F.; Lanciotti, M.; Marcolín, A. Importancia Forestal de los Suelos Volcánicos de la Patagonia Norte y Central; Instituto Nacional de Tecnología Agropecuaria: Bariloche, Argentina, 1995; p. 28. [Google Scholar]
- La Manna, L.; Buduba, C.G.; Irisarri, J. Suelos volcánicos de la provincia del Chubut. In Suelos y Vulcanismo; Imbelloni, P., Barbosa, O., Eds.; Asociación Argentina de la Ciencia del Suelo: Buenos Aires, Argentina, 2020; pp. 333–360. [Google Scholar]
- Troncoso, O. Histología de la afección de Phytophthora austrocedri en los tejidos de conducción de Austrocedrus chilensis. Ph.D. Thesis, Universidad Nacional de la Patagonia San Juan Bosco, Comodoro Rivadavia, Argentina, May 2018. [Google Scholar]
- Mc Neely, J.A. The Great Reshuffling: Human Dimensions of Invasive Alien Species; The Word Conservation Union: Cape Town, South Africa, 2001; p. 242. [Google Scholar]
- Liebhold, A.M.; Brockerhoff, E.G.; Garrett, L.J.; Parke, J.L.; Britton, K.O. Live plant imports: The major pathway for forest insect and pathogen invasions of the US. Front. Ecol. Environ. 2012, 10, 135–143. [Google Scholar] [CrossRef]
- Dale, A.; Feau, N.; Ponchart, J.; Bilodeau, G.; Berube, J.; Hamelin, R.C. Urban activities influence on Phytophthora species diversity in British Columbia, Canada. In Proceedings of the Sudden Oak Death Sixth Science Symposium. Gen. Tech. Rep. GTR-PSW-255, San Francisco, CA, USA, 20–23 June 2016. [Google Scholar]
- Swiecki, T.J.; Bernhardt, E. Best Management Practices for Preventing Phytophthora Introduction and Spread: Trail Work, Construction, Soil Import. 2018. Available online: http://phytosphere.com/publications/Phytosphere_GGNPC_Soil_Phytophthora_BMPs_Jan2018.pdf (accessed on 21 October 2020).
- Chauchard, L. Evaluación del Riesgo de Caída de Árboles en Áreas Recreativas. Región Patagonia; Administración de Parques Nacionales: Buenos Aires, Argentina, 2017; p. 80. [Google Scholar]
- Goheen, D.J.; Mallams, K.; Betlejewski, F.; Hansen, E. Effectiveness of Vehicle Washing and Roadside Sanitation in Decreasing Spread Potential of Port-Orford-Cedar Root Disease. West. J. Appl. For. 2012, 27, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Colquhoun, I.J.; Hardy, G.E. Managing the risks of Phytophthora root and collar rot during bauxite mining in the Eucalyptus marginata (Jarrah) forest of Western Australia. Plant Dis. 2000, 84, 116–127. [Google Scholar] [CrossRef] [Green Version]







| Variable | Percent Contribution |
|---|---|
| Distance to houses | 41.6 |
| Distance to roads | 23.8 |
| Distance to watercourses | 12.5 |
| MAP | 7.4 |
| South | 5.8 |
| Altitude | 4.7 |
| Slope | 3.9 |
| East | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordana, G.; Kitzberger, T.; La Manna, L. Anthropogenic Factors Control the Distribution of a Southern Conifer Phytophthora Disease in a Peri-Urban Area of Northern Patagonia, Argentina. Forests 2020, 11, 1183. https://0-doi-org.brum.beds.ac.uk/10.3390/f11111183
Giordana G, Kitzberger T, La Manna L. Anthropogenic Factors Control the Distribution of a Southern Conifer Phytophthora Disease in a Peri-Urban Area of Northern Patagonia, Argentina. Forests. 2020; 11(11):1183. https://0-doi-org.brum.beds.ac.uk/10.3390/f11111183
Chicago/Turabian StyleGiordana, Guillermo, Thomas Kitzberger, and Ludmila La Manna. 2020. "Anthropogenic Factors Control the Distribution of a Southern Conifer Phytophthora Disease in a Peri-Urban Area of Northern Patagonia, Argentina" Forests 11, no. 11: 1183. https://0-doi-org.brum.beds.ac.uk/10.3390/f11111183