Influence of INGER and TORDIS Energetic Willow Clones Planted on Contaminated Soil on the Survival Rates, Yields and Calorific Value
Abstract
:1. Introduction
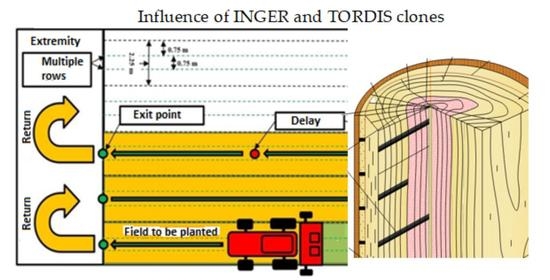
2. Materials and Methods
3. Results and Discussions
3.1. Seedling Survival Rate
3.2. Evaluation of the Amount of Biomass after One Year of Culture
3.3. Soil Enrichment
3.4. Calorific Value
- For the Inger clone: HCV = (18,906–19,846) kJ/kg; LCV = (18,328–19,208) kJ/kg;
- For the Tordis clone: HCV = (18,801–19,909) kJ/kg; LCV = (18,091–19,171) kJ/kg.
4. Conclusions
- Energetic willow can be used in all countries, but it is especially suitable in developing countries where there are uncultivated, arid, contaminated or other lands with low productivity.
- The survival rate was favorable for the Tordis clone, with a 38.4% increase on contaminated land, which recommends the use of the Tordis clone on contaminated land.
- The amount of biomass obtained after the first year of cultivation was 0.64 t/h or 4.03 m3/ha in the case of the Inger clone and 0.66 t/ha or 4.16 m3/ha in the case of the Tordis clone.
- The phytoremediation action of the soil was demonstrated by decreasing the sodium content and increasing the C/N ratio.
- The calorific powers of the two clones were almost similar the obtaining results having reliable intervals with small differences.
- The use of Salix viminalis for the remediation of some contaminated lands represents a beneficial activity both for the land and through the contribution of wood biomass with superior energetic properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Roman, K.; Barwicki, J.; Hryniewicz, M.; Szadkowska, D.; Szadkowski, J. Production of Electricity and Heat from Biomass Wastes Using a Converted Aircraft Turbine AI-20. Processes 2021, 9, 364. [Google Scholar] [CrossRef]
- Stocker, M. Biofuels and biomass-to-liquid fuels in biorefinery: Catalytic conversion of lignocellulosic biomass using porous materials. Angew. Chem. Int. Ed. 2008, 47, 9200–9211. [Google Scholar] [CrossRef]
- Volk, T.A.; Verwijst, T.; Tharakan, P.J.; Abrahamson, L.P.; White, E.H. Growing fuel: A sustainability assessment of willow biomass crops. Front. Ecol. Environ. 2004, 2, 411–418. [Google Scholar] [CrossRef]
- Energetic Salix. Available online: http://www.kwg.ro/files/Salcia_Energetica_pentru_comunitatile_locale.pdf (accessed on 24 January 2021).
- IEA, Renewables. 2020. Available online: https://iea.blob.core.windows.net/assets/f7de18a8-2fca-4df7-ab00-706d509e48c1/Renewables2020_Launch_FINAL_web.pdf (accessed on 24 January 2021).
- Griu (Dobrev), T. Evaluation and Increase of Calorific Value for Wooden Biomass. Ph.D. Thesis, Transilvania University of Braşov, Brasov, Romania, 2014. [Google Scholar]
- Scriba, C. Research on plantation, harvest and capitalization of some energy willow clones. Ph.D. Thesis, Transilvania University of Brasov, Brasov, Romania, 2017. [Google Scholar]
- Griu, T.; Lunguleasa, A. Salix viminalis vs. Fagus sylvatica—fight for renewable energy from woody biomass in Romania. Environ. Eng. Manag. J. 2016, 15, 413–420. [Google Scholar]
- Scriba, C.; Lunguleasa, A.; Salca, E.A.; Ciobanu, V.D. Properties of biomass obtained from short-rotation Inger willow clone grown on a contaminated and non-contaminated land. Maderas. Cienc. Tecnol. 2020, 23. [Google Scholar] [CrossRef]
- Mleczek, M.; Rutowski, P.; Rissman, I.; Kaczmarek, Z.; Golinski, P.; Szentner, K.; Strazynska, K.; Stachowiak, A. Biomass productivity and phytoremediation potential of Salix alba and Salix viminalis. Biomass Bioenergy 2010, 34, 1410–1418. [Google Scholar] [CrossRef]
- Sobczyk, W.; Sobczyk, E.J. Economical and ecological effects of cultivation of basket willow Salix viminalis L. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 2nd International Conference on the Sustainable Energy and Environmental Development, Beijing, China, 24–25 March 2019; IOP Publishing: Bristol, UK, 2019; Volume 214, p. 012002. [Google Scholar] [CrossRef]
- Botu, I.; Botu, M.; Preda, S.; Achim, G.; Lazar, A.; Alecu, A. Comparative evaluation of Romanian and introduced Salix cultivars for short rotation coppice. South West. J. Hortic. Biol. Environ. 2013, 4, 35–42. [Google Scholar]
- Börjesson, P.; Berndes, G. The prospects for willow plantation for wastewater treatment in Sweden. Biomass Bioenergy 2006, 30, 428–438. [Google Scholar] [CrossRef]
- Borišev, M.; Pajević, S.; Nikolić, N.; Orlović, S.; Župunski, M.; Pilipović, A.; Kebert, M. Magnesium and iron deficiencies alter Cd accumulation in Salix viminalis L. Int. J. Phytoremediat. 2016, 18, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Ens, J.; Farrell, R.; Bélanger, N. Early effects of afforestation with willow (Salix purpurea, “Hotel”) on soil carbon and nutrient availability. Forests 2013, 4, 137–154. [Google Scholar] [CrossRef]
- Holm, B.; Heinsoo, K. Municipal wastewater application to Short Rotation Coppice of willows—Treatment efficiency and clone response in Estonian case study. Biomass Bioenergy 2013, 57, 126–135. [Google Scholar] [CrossRef]
- Tőzsér, D.; Harangi, S.; Baranyai, E.; Lakatos, G.; Fülöp, Z.; Tóthmérész, B.; Simon, E. Phytoextraction with Salix viminalis in a moderately to strongly contaminated area. Environ. Sci. Pollut. Res. 2018, 25, 3275–3290. [Google Scholar] [CrossRef]
- Ericsson, K.; Rosenqvist, H.; Ganko, E.; Pisarek, M.; Nilsson, L. An agroeconomic analysis of willow cultivation in Poland. Biomass Bioenergy 2006, 30, 16–27. [Google Scholar] [CrossRef]
- Helby, P.; Rosenqvist, H.; Roos, A. Retreat from Salix—Swedish experience with energy crops in the 1990s. Biomass Bioenergy 2006, 30, 422–427. [Google Scholar] [CrossRef]
- Saletnik, B.; Puchalski, C. Suitability of Biochar and Biomass Ash in Basket Willow (Salix viminalis L.) Cultivation. Agronomy 2019, 9, 577. [Google Scholar] [CrossRef] [Green Version]
- Gąsecka, M.; Mleczek, M.; Drzewiceka, K.; Magdziak, Z.; Rissmann, I.; Chadzinikolau, T.; Golinski, P. Physiological and morphological changes in Salix viminalis L. as a result of plant exposure to copper. J. Environ. Sci. Health Part A 2012, 47. [Google Scholar] [CrossRef]
- Smaliukas, D.; Noreika, R.; Puida, E. Evaluation of morphological biomass and energetic characteristics of Salix viminalis L. and S. dasyclados Wimm. Biologija 2008, 54, 97–100. [Google Scholar] [CrossRef] [Green Version]
- Amichev, B.Z.; Hangs, R.D.; Van Ress, K.C.J. A novel approach to simulate the growth of multi-stem willow in Bioenergy production systems with a simple process-based model (3PG). Biomass Bioenergy 2011, 35, 473–488. [Google Scholar] [CrossRef]
- Argus, G.W. Infrageneric classification of Salix (Salicaceae) in the New World. Syst. Bot. Monogr. 1997, 52, 1–121. [Google Scholar] [CrossRef]
- Krajnic, N. Wood Fuels Handbook, Pristina 2015; Food and Agriculture Organization (FAO) of United Nations (Rome, Italy). Available online: http://large.stanford.edu/courses/2017/ph240/timcheck1/docs/fao-krajnc-2015.pdf (accessed on 18 February 2021).
- DIN 51900-1. Determining the Gross Calorific Value of Solid and Liquid Fuels Using the Bomb Calorimeter, and Calculation of Net Calorific Value—Part 1: General Information; Deutsches Institute fur Normung: Berlin, Germany, 2000. [Google Scholar]
- Albertsson, J.; Verwijst, T.; Hansson, D.; Bertholdsson, N.-O.; Åhman, I. Effects of competition between short-rotation willow and weeds on performance of different clones and associated weed flora during the first harvesting cycle. Biomass Bioenergy 2014, 70, 364–372. [Google Scholar] [CrossRef]
- Dimitrious, I.; Rosenqvist, H.; Berndes, G. Slow expansion and low yields of willow short rotation coppice in Sweden; implications for future strategies. Biomass Bioenergy 2011, 35, 4613–4618. [Google Scholar] [CrossRef]
- El Kasmioui, O.; Ceulemans, R. Financial analysis of the cultivation of short rotation woody crops for Bioenergy in Belgium: Barriers and opportunities. Bioenergy Res. 2013, 6, 336–350. [Google Scholar] [CrossRef]
- Arevalo, C.B.M.; Volk, T.A.; Bevilacqua, E.; Abrahamson, L. Development and validation of aboveground biomass estimations for four Salix clones in central New York. Biomass Bioenergy 2007, 31, 1–12. [Google Scholar] [CrossRef]
- Borkowska, H.; Molas, R. Yield comparison of four lignocellulosic perennial energy crop species. Biomass Bioenergy 2013, 51, 145–153. [Google Scholar] [CrossRef]
- Buchholz, T.; Volk, T.A. Improving the profitability of willow crops -identifying opportunities with a crop budget model. Bioenergy Res. 2011, 4, 85–95. [Google Scholar] [CrossRef]
- Ericsson, K.; Nilsson, L.J. Assessment of the potential biomass supply in Europe using a resource-focused approach. Biomass Bioenergy 2006, 30, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Adler, A.; Verwijst, T.; Aronsson, P. Estimation and relevance of bark proportion in a willow stand. Biomass Bioenergy 2005, 29, 102–113. [Google Scholar] [CrossRef]
- Faasch, R.J.; Patenaude, G. The economics of short rotation coppice in Germany. Biomass Bioenergy 2012, 45, 27–40. [Google Scholar] [CrossRef]
- Fiala, M.; Bacenetti, J. Economic, energetic and environmental impact in short rotation coppice harvesting operations. Biomass Bioenergy 2012, 42, 107–113. [Google Scholar] [CrossRef]
- Fischer, G.; Prieler, S.; van Velthuizen, H. Biomass potentials of miscanthus, willow and poplar: Results and policy implication for Eastern Europe, Northern and Central Asia. Biomass Bioenergy 2005, 28, 119–132. [Google Scholar] [CrossRef]
- Sevel, L.; Nord-Larsen, T.; Raulund-Rasmussen, H. Biomass production of four willow clones grown as short rotation coppice on two soil types in Denmark. Biomass Bioenergy 2012, 46, 664–672. [Google Scholar] [CrossRef]
- Stolarski, M.; Szczukowski, S.; Tworkowski, J.; Klasa, A. Productivity of seven clones of willow coppice in annual and quadrennial cutting cycles. Biomass Bioenergy 2008, 32, 1227–1234. [Google Scholar] [CrossRef]
- Szczukowski, S.; Tworkowski, J.; Klasa, A.; Stolarski, M. Productivity and chemical composition of wood tissues of short rotation willow coppice cultivated on arable land. Rostl. Vyrob. 2002, 48, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; MacFarlane, D.W. Evaluating the biomass production of coppiced willow and polar clones in Michigan, USA, over multiple rotations and different growing conditions. Biomass Bioenergy 2012, 46, 380–388. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Evans, E.J.; Bilsborrow, P.E.; Wright, C.; Wewison, W.O.; Pilbeam, D.J. Yield of willow cultivars at different planting densities in a commercial short rotation coppice in the north of England. Biomass Bioenergy 2007, 31, 469–474. [Google Scholar] [CrossRef]
- Ghaley, B.B.; Porter, J.R. Determination of biomass accumulation in mixed belts of Salix, Corylus and Alnus species in combined food and energy production system. Biomass Bioenergy 2014, 63, 86–91. [Google Scholar] [CrossRef]
- Guidi, W.; Piccioni, E.; Ginanni, M.; Bonari, E. Bark content estimation in poplar (Populus deltoides L.) short-rotation coppice in Central Italy. Biomass Bioenergy 2008, 32, 518–524. [Google Scholar] [CrossRef]
- Hammar, T.; Ericsson, N.; Sundberg, C.; Hannson, P.A. Climate impact of willow grown for energy in Sweden. Bioenergy Res. 2014, 7, 1529–1540. [Google Scholar] [CrossRef] [Green Version]
- Krigstin, S.G.; Wong, B.M.; Roy, D.N. The contribution of chemical components in juvenile hybrid Salix spp. to its total energy output. Wood Sci. Technol. 1993, 27, 309–320. [Google Scholar] [CrossRef]
- Labrecque, M.; Teodorescu, T.I. Field performance and biomass production of 12 willow and poplar clones in short-rotation coppice in southern Quebec (Canada). Biomass Bioenergy 2005, 29, 1–9. [Google Scholar] [CrossRef]
- Larsen, S.U.; Jørgensen, U.; Lærke, P.E. Willow yield is highly dependent on clone and site. Bioenergy Res. 2014, 7, 1280–1292. [Google Scholar] [CrossRef]
- Hangs, R.D.; Van Rees, K.C.J.; Shoenau, J.J.; Guo, X. A simple technique for estimating above-ground biomass in short-rotation plantations. Biomass Bioenergy 2011, 35, 2156–2162. [Google Scholar] [CrossRef]
- Jezowski, S.; Glowacka, K.; Kaczmarek, Z.; Szuczukowski, S. Yield traits of eight common Osier clones in the first three years following planting in Poland. Biomass Bioenergy 2011, 35, 1205–1210. [Google Scholar] [CrossRef]
- Karp, A.; Hanley, S.; Trybush, S.; Macalpine, W.; Pei, M.; Shield, I. Genetic Improvement of Willow for Bioenergy and Biofuels. J. Integr. Plant Biol. 2011, 53, 151–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzone, M.; Balsari, P. Planters performance during a Very Short Rotation Coppice planting. Biomass Bioenergy 2014, 67, 188–192. [Google Scholar] [CrossRef]
- Mitsui, Y.; Seto, S.; Nishio, M.; Minato, K.; Ishizawa, K.; Satoh, S. Willow clones with high biomass yield in short rotation coppice in the southern region of Tohoku district (Japan). Biomass Bioenergy 2010, 34, 467–473. [Google Scholar] [CrossRef]
- Rytter, R.M. The potential of willow and poplar plantations as carbon sinks in Sweden. Biomass Bioenergy 2012, 36, 86–95. [Google Scholar] [CrossRef]











| No. | Soil Features | Before Planting | After Two Year of Planting |
|---|---|---|---|
| 1 | pH | 8.1 | 8.3 |
| 2 | Humus content | 2.76 | 3.60 |
| 3 | C/N ratio | 11.53 | 13.06 |
| 4 | Sodium, ppm | 188.5 | 35.21 |
| 5 | Carbon content, ppm | 1.60 | 2.09 |
| 6 | P content, ppm | 25 | 80 |
| 7 | K content, ppm | 76.33 | 107.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scriba, C.; Lunguleasa, A.; Spirchez, C.; Ciobanu, V. Influence of INGER and TORDIS Energetic Willow Clones Planted on Contaminated Soil on the Survival Rates, Yields and Calorific Value. Forests 2021, 12, 826. https://0-doi-org.brum.beds.ac.uk/10.3390/f12070826
Scriba C, Lunguleasa A, Spirchez C, Ciobanu V. Influence of INGER and TORDIS Energetic Willow Clones Planted on Contaminated Soil on the Survival Rates, Yields and Calorific Value. Forests. 2021; 12(7):826. https://0-doi-org.brum.beds.ac.uk/10.3390/f12070826
Chicago/Turabian StyleScriba, Cezar, Aurel Lunguleasa, Cosmin Spirchez, and Valentina Ciobanu. 2021. "Influence of INGER and TORDIS Energetic Willow Clones Planted on Contaminated Soil on the Survival Rates, Yields and Calorific Value" Forests 12, no. 7: 826. https://0-doi-org.brum.beds.ac.uk/10.3390/f12070826