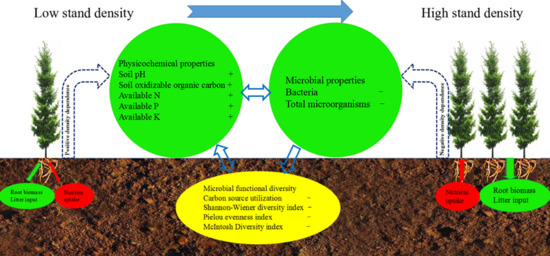
Unravelling the Functional Diversity of the Soil Microbial Community of Chinese Fir Plantations of Different Densities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Profile of the Study Site and Soil Collection
2.2. Soil Physicochemical Analysis
2.3. Soil Microbial Flora and Metabolic Activity Analysis
2.4. Statistic Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Soil Microbial Flora and Metabolic Activities
3.3. Effects of Physicochemical Factors on Carbon Substrates Utilization
4. Discussion
4.1. Shift in the Soil Physicochemical Factors
4.2. Shift in Soil Microbial Flora and Metabolic Activities
4.3. Effects of Physicochemical Factors on Functional Diversity of Microbial Community
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mummey, D.L.; Stahl, P.D.; Buyer, J.S. Microbial biomarkers as an indicator of ecosystem recovery following surface mine reclamation. Appl. Soil Ecol. 2002, 21, 251–259. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Qu, L.Y.; Chen, L.D. An Amendment on Information Extraction of Biolog EcoPlateTM. Microbiology 2009, 36, 1083–1091. [Google Scholar]
- Harris, J.A. Measurements of the soil microbial community for estimating the success of rest oration. Eur. J. Soil Sci. 2003, 54, 801–808. [Google Scholar] [CrossRef]
- Wu, C.W.; Zhao, L.P. Technologies on soil microbiology diversity. Chin. Agric. Sci. Bull. 2011, 27, 231–235. [Google Scholar]
- Carter, M.R.; Gregorich, E.G.; Angers, D.A.; Beare, M.H.; Sparling, G.P.; Wardle, D.A.; Voroney, R.P. Interpretation of microbial biomass measurements for soil quality assessment in humid temperate regions. Can. J. Soil Sci. 1999, 79, 507–520. [Google Scholar] [CrossRef] [Green Version]
- Winding, A.; Hund-Rinke, K.; Rutgers, M. The use of microorganisms in ecological soil classification and assessment concepts. Ecotoxicol. Environ. Saf. 2005, 62, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Crecchio, C.; Gelsomino, A.; Ambrosoli, R.; Minati, J.L.; Ruggiero, P. Functional and molecular responses of soil microbial communities under differing soil management practices. Soil Biol. Biochem. 2004, 36, 1873–1883. [Google Scholar] [CrossRef]
- Oren, A.; Steinberger, Y. Catabolic profles of soil fungal communities along a geographic climatic gradient in Israel. Soil Biol. Biochem. 2008, 40, 2578–2587. [Google Scholar] [CrossRef]
- Smalla, K.; Wachtendorf, U.; Heuer, H.; Liu, W.T.; Forney, L. Analysis of biolog gn substrate utilization patterns by microbial communities. Appl. Environ. Microbiol. 1998, 64, 1220–1225. [Google Scholar] [PubMed]
- Garland, J.L.; Mills, A. Classification and characterisation of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar] [PubMed]
- Zak, J.C.; Willig, M.R.; Moorhead, D.L.; Wildman, H.G. Functional diversity of microbial communities: A quantitative approach. Soil Biol. Biochem. 1994, 26, 1101–1108. [Google Scholar] [CrossRef]
- Ji, L.B.; Shu, D.W. Influence of stand density of cunninghamia lanceolate on stand growth. For. Inv. Plan. 2017, 42, 135–137. [Google Scholar]
- Chen, J.; Duan, B.L.; Wang, M.L.; Korpelainen, H.; Li, C.Y. Intra- and inter-sexual competition of Populus cathayana under different watering regimes. Funct. Ecol. 2014, 28, 124–136. [Google Scholar] [CrossRef]
- Dybzinski, R.; Farrior, C.E.; Ollinger, S.; Pacala, S.W. Interspecific vs intraspecific patterns in leaf nitrogen of forest trees across nitrogen availability gradients. New Phytol. 2013, 200, 112–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, T.; Freckleton, R.P.; Lewis, O.T. Plant pathogens drive density-dependent seedling mortality in a tropical tree. Ecol. Lett. 2006, 9, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Terborgh, J. Enemies maintain hyperdiverse tropical forests. Am. Nat. 2012, 179, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Kos, M.; Veendrick, J.; Bezemer, T.M. Local variation inconspecific plant density influences plant-soil feedback in a natural grassland. Basic Appl. Ecol. 2013, 14, 506–514. [Google Scholar] [CrossRef]
- Comita, L.S.; Queenborough, S.A.; Murphy, S.J.; Eck, J.L.; Xu, K.; Krishnadas, M.; Beckman, N.; Zhu, Y. Testing predictions of the Janzen–Connell hypothesis: A meta-analysis of experimental evidence for distance-and density-dependent seed and seedling survival. J. Ecol. 2014, 102, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.Y.; Yang, X.J.; Li, X.; Ren, L.N. Soil organic carbon and nutrients in natural Larix olgensis at different stand densities. J. Northeast For. Univ. 2013, 41, 51–55. [Google Scholar]
- Zhao, R.D.; Fan, J.B.; He, Y.Q.; Song, C.L.; Tu, R.F.; Tan, B.C. Effects of stand density on soil nutrients and enzyme activity under Pinus massoniana forest. Soils 2012, 44, 297–301. [Google Scholar]
- Barron-Gafford, G.A.; Will, R.E.; Burkes, E.C. Nutrient concentrations and contents and their relation to stem growth of intensively managed Pinus taeda and Pinus elliottii stands of different planting densities. For. Sci. 2003, 49, 291–300. [Google Scholar]
- Chen, H. Phosphatase activity and P fractions in soils of an 18-year-old Chinese fir (Cunninghamia lanceolata) plantation. For. Ecol. Manag. 2003, 178, 301–310. [Google Scholar] [CrossRef]
- Chen, H.H. Effects of density regulation on undergrowth vegetation and soil fertility of Cunninghamia lanceolata plantation. Sci. Silvae Sin. 2017, 28, 88–90. [Google Scholar]
- Tian, D.L.; Xiang, W.H.; Chen, X.Y.; Yan, W.D.; Fang, X.; Kang, W.X.; Dan, X.W.; Peng, C.H.; Peng, Y.Y. A long-term evaluation of biomass production in first and second rotations of Chinese fir plantations at the same site. Forestry 2011, 84, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Wang, J.; Di, Y.; Wang, H. Differences in fine-root biomass of trees and understory vegetation among stand types in subtropical forests. PLoS ONE 2015, 10, e0128894. [Google Scholar] [CrossRef] [PubMed]
- Du, H.L. Density Effect of Soil Nutrientand Stoichiometry of Cunninghamia lanceolata Plantationsin Naxi, Sichuan Province; Chinese Academy of Forestry: Beijing, China, 2017; pp. 15–16. [Google Scholar]
- Institute of Soil Science, Chinese Academy of Sciences. Soil Physical and Chemical Analysis; Shanghai Scientific & Technical Publishers: Shanghai, China, 1978. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; America Society of Agronomy: Madison, WI, USA, 1982; pp. 539–579. ISBN 0891188266. [Google Scholar]
- Tirol-Padre, A.; Ladha, J.K. Assessing the reliability of permanganate-oxidizable carbon as an index of soil labile carbon. Soil Sci. Soc. Am. J. 2004, 68, 969–978. [Google Scholar] [CrossRef]
- Classen, A.T.; Boyle, S.I.; Haskins, K.E.; Overby, S.T.; Hart, S.C. Community-level physiological profiles of bacteria and fungi: Plate type and incubation temperature influences on contrasting soils. FEMS Microbiol. Ecol. 2003, 44, 319–328. [Google Scholar] [CrossRef]
- Degens, B.P.; Schipper, L.A.; Sparling, G.P.; Vukovic, M.V. Decreases in organic C reserves in soils can reduce the catabolic diversity of soil microbial communities. Soil Biol. Biochem. 2000, 32, 189–196. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pielou, E.C. Mathematical ecology. J. Anim. Ecol. 1975, 47, 347–351. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163–688. [Google Scholar] [CrossRef]
- Yang, Y.H.; Yao, J.; Hu, X.M. Effect of pesticide pollution against functional microbial diversity in soil. J. Microbiol. 2000, 20, 23–25. [Google Scholar]
- Choi, K.H.; Dobbs, F.C. Comparison of two kinds of Biolog micro-plates (GN and ECO) in their ability to distinguish among aquatic microbial communities. J. Microbiol. Meth. 1993, 36, 203–213. [Google Scholar] [CrossRef]
- Lu, S.B.; Zhang, Y.J.; Chen, C.R.; Xu, Z.H.; Guo, X.M. Analysis of functional differences between soil bacterial communities in three different types of forest soils based on biology fingerprint. Acta Pedol. Sin. 2013, 50, 618–623. [Google Scholar]
- Tong, S.Z.; Sheng, W.T.; Zhang, J.G. Study on density effect of Chinese Fir plantation. For. Res. 2002, 15, 66–75. [Google Scholar]
- Bagchi, R.; Swinfield, T.; Gallery, R.E.; Lewis, O.T.; Gripenberg, S.; Narayan, L.; Freckleton, R.P. Testing the JanzenConnell mechanism: Pathogens cause overcompensating density dependence in a tropical tree. Ecol. Lett. 2010, 13, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Dudenhöffer, J.H.; Ebeling, A.; Klein, A.M.; Wagg, C. Beyond biomass: Soil feedbacks are transient over plant life stages and alter fitness. J. Ecol. 2018, 106, 230–241. [Google Scholar] [CrossRef]
- Wei, X.; Bezemer, T.M.; Berendse, F. Density-dependency and plant-soil feedback: Former plant abundance influences competitive interactions between two grassland plant species through plant-soil feedbacks. Plant Soil 2018, 428, 1–12. [Google Scholar]
- Qu, Y.D.; Su, Z.Y.; Peng, G.X.; Liu, G. Soil microbial functional diversity in a montane evergreen broadleaved forest of Chebaling following the huge ice storm in south China. Acta Ecol. Sin. 2009, 29, 6156–6164. [Google Scholar]
- Tian, Q.; Wang, X.; Wang, D.; Wang, M.; Liao, C.; Yang, X. Decoupled linkage between soil carbon and nitrogen mineralization among soil depths in a subtropical mixed forest. Soil Biol. Biochem. 2017, 109, 135–144. [Google Scholar] [CrossRef]
- Mediavilla, S.; Escudero, A.; Heilmeier, H. Internal leaf anatomy and photosynthetic resource-use efficiency: Interspecific and intraspecific comparisons. Tree Physiol. 2001, 21, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Showalter, A.M.; Ungar, I.A. Effects of intraspecific competition on growth and photosynthesis of Atriplex prostrata. Aquat. Bot. 2005, 83, 187–192. [Google Scholar] [CrossRef]
- Miki, T. Microbe-mediated plant–soil feedback and its roles in a changing world. Ecol. Res. 2012, 27, 509–520. [Google Scholar] [CrossRef]
- Miki, T.; Ushio, M.; Fukui, S.; Kondoh, M. Functional diversity of microbial decomposers facilitates plant coexistence in a plant-microbe-soil feedback model. Proc. Natl. Acad. Sci. USA 2010, 107, 14251–14256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.S.; Lin, X.G.; Zhang, H.Y.; Yin, R.; Duan, Z.Q.; Shi, W.M. Microbial activity and functional diversity in soils used for the commercial production of cucumbers and tomatoes in polytunnel greenhouse, under different fertilization. Acta Ecol. Sin. 2008, 28, 2685–2686. [Google Scholar]
- Acosta-Martínez, V.; Dowd, S.E.; Sun, Y.; Wester, D.; Allen, V. Pyrosequencing analysis for characterization of soil bacterial populations as affected by an integrated livestock-cotton production system. Appl. Soil. Ecol. 2010, 45, 13–25. [Google Scholar] [CrossRef]
- Kennedy, A.C. Microbial diversity in agroecosystem quality. In Biodiversity in Agroecosystems; Collins, W.W., Qualset, C.O., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 1–17. ISBN 978-1-13802-781-7. [Google Scholar]
- Wang, S.L.; Chen, L.C.; Liao, L.P.; Huang, Z.Q. Effects of three kinds of allelochemicals on growth of Chinese fir seedlings. Chin. J. Appl. Environ. Biol. 2002, 8, 588–591. [Google Scholar]
- Wang, Q.; Shi, C.J.; Zhou, D.Q.; Liu, Y.J.; Wang, B.Y.; Sun, J.X. Promotion effect of recycled water irrigation on microorganism quantity in urban greenspace soil. J. Southwest For. Univ. 2012, 32, 12–16. [Google Scholar]
- Zhao, G.C.; Liang, J.; Dan, Y.J.; Wang, J.; Qin, Y.; Zhang, W.H. Review of studies on relationship between soil microbes and plants. J. Southwest For. Univ. 2011, 31, 83–87. [Google Scholar]
- Zhao, W.N.; Wang, Y.X.; Chen, Q.B.; Nie, L.; Yang, Y.Y. Effect of soil physical-chemical properties and microorganism quantity on enzyme activity in natural evergreen broad-leaved forest by path analysis. J. Northeast For. Univ. 2016, 44, 75–80. [Google Scholar]
- Grove, J.A.; Kautola, H.; Javadpour, S.; Moo-Young, M.; Anderson, W.A. Assessmentof changes in the microorganism community in a biofilter. Biochem. Eng. J. 2004, 18, 111–114. [Google Scholar] [CrossRef]
- Hadwin, A.M.; Del Rio, L.F.; Pinto, L.J.; Painter, M.; Routledge, R.; Moore, M.M. Microbial communities in wetlands of the Athabasca oil sands: Geneticand metabolic characterization. FEMS Microbiol. Ecol. 2006, 55, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Zhen, H.; Ou, Y.Z.; Fang, Z.G.; Zhao, T.Q. Application of BIOLOG to study on soil microbial community functional diversity. Acta Pedol. Sin. 2004, 41, 456–461. [Google Scholar]
- Jonathan, Z.K.; John, A.B. High incidence of halotolerant bacteria in Pacifc hydrothermal-vent and pelagic environments. FEMS Microbiol. Ecol. 2000, 32, 249–260. [Google Scholar]
- O’Donnell, A.G.; Seasman, M.; Macrae, A.; Waite, I.; Davies, J.T. Plants and fertilizers as drivers of change in microbial community structure and function in soils. Plant Soil 2001, 232, 135–145. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Tardif, J.C.; Adkins, A.; Staniforth, R. Functional diversity of microbial communities in the mixed boreal plain forest of central Canada. Soil Biol. Biochem. 2005, 37, 1359–1372. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, X.; Tan, Y.; Liu, H.L.; Tian, Z.P.; Zeng, G.P. Dynamics between soil microorganism and soil character actors during Carthamus tinctorius growth periods. Pratacult. Sci. 2011, 28, 2084–2091. [Google Scholar]




| Density | D1 | D2 | D3 | D4 | D5 |
|---|---|---|---|---|---|
| Initial density (stems∙hm−2) | 1667 | 3333 | 5000 | 6667 | 10,000 |
| Existing density (stems∙hm−2) | 1578 | 2294 | 2617 | 2789 | 2461 |
| Index | Formula | Definitions | Reference |
|---|---|---|---|
| Average well color development (AWCD) | C is the difference of the two-band optical density of each carbon source well; R is the optical density value of the control well; n is the number of carbon source species in the medium (31 in this study) | [31] | |
| Shannon-Wiener diversity index (H’) | H’ = −ΣPi ln Pi | Pi represents the ratio of the absorbance in the i-th non-control well to the sum of the absorbance of all non-control wells | [32] |
| Pielou evenness index (J) | S is the total number of carbon sources utilized | [33] | |
| Simpson dominance index (D) | D = 1 − ∑Pi2. | [34] | |
| McIntosh diversity index (U) | ni is the (C − R) value of the i-th well | [35] | |
| McIntosh evenness index (E) | N is the sum of the (C − R) values | [35] |
| Density | pH | SM | BD | OM | SOOC | TN | AN | AP | AK |
|---|---|---|---|---|---|---|---|---|---|
| (%) | (g/cm3) | (g/kg) | (g/kg) | (g/kg) | (mg/kg) | (mg/kg) | (mg/kg) | ||
| D1 | 5.04 ± 0.02b | 35.72 ± 2.31a | 1.29 ± 0.08a | 42.80 ± 2.60a | 5.02 ± 0.63c | 1.61 ± 0.05a | 133.00 ± 4.04a | 2.09 ± 0.57b | 78.61 ± 4.23b |
| D2 | 5.07 ± 0.02b | 30.92 ± 2.62a | 1.34 ± 0.08a | 41.32 ± 2.53a | 5.27 ± 0.91c | 1.56 ± 0.04a | 129.85 ± 6.17a | 2.15 ± 0.28b | 85.96 ± 5.24ab |
| D3 | 5.29 ± 0.01ab | 32.37 ± 1.49a | 1.32 ± 0.03a | 41.55 ± 1.64a | 7.61 ± 0.16a | 1.55 ± 0.04a | 140.23 ± 7.12a | 2.53 ± 0.53ab | 109.24 ± 6.25a |
| D4 | 5.49 ± 0.02a | 34.23 ± 0.58a | 1.31 ± 0.01a | 41.53 ± 0.69a | 7.63 ± 0.84a | 1.57 ± 0.09a | 148.40 ± 4.45a | 2.68 ± 0.18a | 126.24 ± 5.28a |
| D5 | 5.27 ± 0.04ab | 32.61 ± 1.96a | 1.31 ± 0.05a | 39.47 ± 1.46a | 6.67 ± 0.61b | 1.52 ± 0.05a | 143.77 ± 10.17a | 2.67 ± 0.60a | 95.97 ± 6.24ab |
| Density | Bacteria Quantity (107 cfu/g) | Fungi Quantity (105 cfu/g) | Actinomycete Quantity (106 cfu/g) | Total Microbe Quantity (107 cfu/g) |
|---|---|---|---|---|
| D1 | 3.13 ± 0.09a | 1.07 ± 0.05a | 2.77 ± 0.21ab | 3.42 ± 0.11a |
| D2 | 2.87 ± 0.57a | 0.57 ± 0.01c | 2.30 ± 0.08c | 3.10 ± 0.58a |
| D3 | 2.67 ± 0.50a | 0.70 ± 0.02bc | 2.40 ± 0.33bc | 2.91 ± 0.51a |
| D4 | 2.50 ± 0.33a | 1.17 ± 0.17a | 3.07 ± 0.09a | 2.82 ± 0.33a |
| D5 | 2.77 ± 0.17a | 0.97 ± 0.01ab | 2.33 ± 0.12c | 3.01 ± 0.18a |
| Density | H’ | J | D | U | E |
|---|---|---|---|---|---|
| D1 | 2.41 ± 0.21a | 0.71 ± 0.06a | 0.97 ± 0.01a | 2.24 ± 0.01a | 0.92 ± 0.01a |
| D2 | 2.39 ± 0.26a | 0.70 ± 0.07a | 0.97 ± 0.01a | 2.08 ± 0.01b | 0.93 ± 0.01a |
| D3 | 2.24 ± 0.21a | 0.66 ± 0.06a | 0.97 ± 0.01a | 1.98 ± 0.05c | 0.93 ± 0.01a |
| D4 | 1.75 ± 0.14b | 0.52 ± 0.04b | 0.98 ± 0.01a | 1.50 ± 0.04d | 0.92 ± 0.01a |
| D5 | 2.21 ± 0.17a | 0.65 ± 0.05a | 0.98 ± 0.01a | 1.94 ± 0.06c | 0.93 ± 0.01a |
| Carbon Substrate Type | Carbon Substrate | PC1 (41.92%) | PC2 (19.36%) |
|---|---|---|---|
| Carbohydrates | β-Methyl-d-Glucoside | 4.026 | −0.021 |
| d-Galactonic Acid Lactone | 0.415 | 0.730 | |
| d-Xylose | 3.943 | −0.080 | |
| d-Galacturonic Acid | −7.138 | −0.718 | |
| I-Erythritol | 2.777 | 0.323 | |
| d-Mannitol | −4.342 | −1.166 | |
| N-Acetyl-d-Glucosamine | −4.099 | −1.845 | |
| d-Glucosaminic Acid | −3.179 | −1.229 | |
| d-Cellobiose | 2.295 | 2.074 | |
| α-d-Glucose-1-Phosphate | 3.178 | −0.972 | |
| α-d-Lactose | 4.026 | −0.085 | |
| d,l-α-Glycerol Phosphate | 1.131 | −1.952 | |
| Average value of absolute load value | 3.379 | 0.933 | |
| Anmino acids | l-Arginine | −0.712 | 0.297 |
| l-Asparagine | −6.310 | −0.365 | |
| l-Phenylalanine | −0.186 | −0.132 | |
| l-Serine | −2.994 | 2.193 | |
| l-Threonine | 2.511 | −0.557 | |
| Glycyl-l-Glutamic Acid | 2.213 | −0.046 | |
| Average value of absolute load value | 2.488 | 0.598 | |
| Carboxylic acids | Pyruvic Acid Methyl Ester | −2.524 | 0.078 |
| γ-Hydroxybutyric Acid | 3.400 | 0.169 | |
| Itaconic Acid | 0.534 | 0.755 | |
| α-Ketobutyric Acid | 3.338 | −0.268 | |
| d-Malic Acid | 1.651 | −1.369 | |
| Average value of absolute load value | 2.289 | 0.528 | |
| Polymers | Tween 40 | −8.441 | 0.682 |
| Tween 80 | −5.183 | 1.450 | |
| α-Cyclodextrin | 4.015 | −0.071 | |
| Glycogen | 3.726 | −0.121 | |
| Average value of absolute load value | 5.341 | 0.581 | |
| Phenolic acids | 2-Hydroxybenzoic Acid | 2.632 | 0.117 |
| 4-Hydroxybenzoic Acid | −1.800 | 0.404 | |
| Average value of absolute load value | 3.532 | 0.261 | |
| Amines | Phenylethylamine | 0.757 | 1.400 |
| Putrescine | 0.338 | 0.325 | |
| Average value of absolute load value | 0.548 | 0.913 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Xue, L.; Dong, Y.; Wei, Y.; Jiao, R. Unravelling the Functional Diversity of the Soil Microbial Community of Chinese Fir Plantations of Different Densities. Forests 2018, 9, 532. https://0-doi-org.brum.beds.ac.uk/10.3390/f9090532
Wang C, Xue L, Dong Y, Wei Y, Jiao R. Unravelling the Functional Diversity of the Soil Microbial Community of Chinese Fir Plantations of Different Densities. Forests. 2018; 9(9):532. https://0-doi-org.brum.beds.ac.uk/10.3390/f9090532
Chicago/Turabian StyleWang, Chaoqun, Lin Xue, Yuhong Dong, Yihui Wei, and Ruzhen Jiao. 2018. "Unravelling the Functional Diversity of the Soil Microbial Community of Chinese Fir Plantations of Different Densities" Forests 9, no. 9: 532. https://0-doi-org.brum.beds.ac.uk/10.3390/f9090532