The Biodiversity of Saccharomyces cerevisiae in Spontaneous Wine Fermentation: The Occurrence and Persistence of Winery-Strains
Abstract
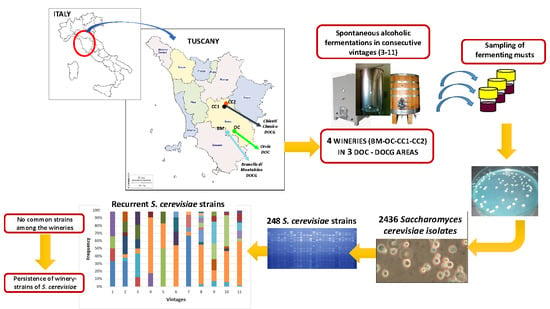
:1. Introduction
2. Materials and Methods
2.1. The Isolation of Saccharomyces cerevisiae from Spontaneous Wine Fermentations
2.2. Genotypic Characterization of S. cerevisiae Isolates
2.3. Data Analyses
3. Results and Discussion
3.1. The Biodiversity of S. cerevisiae Isolates from Different Wineries in Consecutive Vintages
3.2. The Persistence of S. cerevisiae Strains in Different Wineries in Consecutive Vintages
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Bisson, L.F. Geographic origin and diversity of wine strains of Saccharomyces. Am. J. Enol. Vitic. 2012, 63, 165–175. [Google Scholar] [CrossRef]
- Schuller, D.; Casal, M. The genetic structure of fermentative vineyard-associated Saccharomyces cerevisiae populations revealed by microsatellite analysis. Antonie Van Leeuwenhoek 2007, 91, 137–150. [Google Scholar] [CrossRef]
- Combina, M.; Elía, A.; Mercado, L.; Catania, C.; Ganga, A.; Martinez, C. Dynamics of indigenous yeast populations during spontaneous fermentation of wines from Mendoza, Argentina. Int. J. Food Microbiol. 2005, 99, 237–243. [Google Scholar] [CrossRef]
- Mercado, L.; Sturm, M.E.; Rojo, M.C.; Ciklic, I.; Martínez, C.; Combina, M. Biodiversity of Saccharomyces cerevisiae populations in Malbec vineyards from the “Zona Alta del Río Mendoza” region in Argentina. Int. J. Food Microbiol. 2011, 151, 319–326. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Diversity of Saccharomyces cerevisiae yeasts associated to spontaneously fermenting grapes from an Italian “heroic vine-growing area”. Food Microbiol. 2012, 31, 159–166. [Google Scholar] [CrossRef]
- Versavaud, A.; Courcoux, P.; Roulland, C.; Dulau, L.; Hallet, J.N. Genetic diversity and geographical distribution of wild Saccharomyces cerevisiae strains from the wine-producing area of Charentes, France. Appl. Environ. Microbiol. 1995, 61, 3521–3529. [Google Scholar]
- Ganucci, D.; Guerrini, S.; Mangani, S.; Vincenzini, M.; Granchi, L. Quantifying the effects of ethanol and temperature on the fitness advantage of predominant Saccharomyces cerevisiae strains occurring in spontaneous wine fermentations. Front. Microbiol. 2018, 9, 1563. [Google Scholar] [CrossRef]
- Perrone, B.; Giacosa, S.; Rolle, L.; Cocolin, L.; Rantsiou, K. Investigation of the dominance behavior of Saccharomyces cerevisiae strains during wine fermentation. Int. J. Food Microbiol. 2013, 165, 156–162. [Google Scholar] [CrossRef]
- Pérez-Torrado, R.; Rantsiou, K.; Perrone, B.; Navarro-Tapia, E.; Querol, A.; Cocolin, L. Saccharomyces cerevisiae strains: Insight into the dominance phenomenon. Sci. Rep. 2017, 7, 43603. [Google Scholar] [CrossRef]
- Vezinhet, F.; Hallet, J.N.; Valade, M.; Poulard, A. Ecological survey of wine strains by molecular methods of identification. Am. J. Enol. Vitic. 1992, 43, 83–86. [Google Scholar]
- Gutièrrez, A.R.; Santamarìa, R.; Epifanio, S.; Garijo, P.; Lopez, R. Ecology of spontaneous fermentation in one winery during 5 consecutive years. Lett. Appl. Microbiol. 1999, 29, 411–415. [Google Scholar] [CrossRef]
- Sabate, J.; Cano, J.; Querol, A.; Guillamon, J.M. Diversity of Saccharomyces strains in wine fermentations: Analysis for two consecutive years. Lett. Appl. Microbiol. 1998, 26, 452–455. [Google Scholar] [CrossRef]
- Torija, M.; Rozès, N.; Poblet, M.; Guillamón, J.M.; Mas, A. Yeast population dynamics in spontaneous fermentations: Comparison between two different wine-producing areas over a period of three years. Antonie Van Leeuwenhoek 2001, 79, 345–352. [Google Scholar] [CrossRef]
- Lopandic, K.; Gangl, H.; Wallner, E.; Tscheik, G.; Leitner, G.; Querol, A.; Gardner, R.C.; Sterflinger, K.; Prillinger, H. Genetically different wine yeasts isolated from Austrian vine-growing regions influence wine aroma differently and contain putative hybrids between Saccharomyces cerevisiae and Saccharomyces kudriavzevii. FEMS Yeast Res. 2007, 7, 953–965. [Google Scholar] [CrossRef]
- Pramateftaki, P.V.; Lanaridis, P.; Typas, M.A. Molecular identification of wine yeasts at species or strain level: A case study with strains from two vine-growing areas of Greece. J. Appl. Microbiol. 2000, 89, 236–248. [Google Scholar] [CrossRef]
- Börlin, M.; Venet, P.; Claisse, O.; Salin, F.; Legras, J.L.; Masneuf-Pomarede, I. Cellar-associated Saccharomyces cerevisiae population structure revealed high level diversity and perennial persistence at Sauternes wine estates. Appl. Environ. Microbiol. 2016, 82, 2909–2918. [Google Scholar] [CrossRef]
- Cocolin, L.; Pepe, V.; Comitini, F.; Comi, G.; Ciani, M. Enological and genetic traits of Saccharomyces cerevisiae isolated from former and modern wineries. FEMS Yeast Res. 2004, 3, 237–245. [Google Scholar] [CrossRef]
- Capece, A.; Granchi, L.; Guerrini, S.; Mangani, S.; Romaniello, R.; Vincenzini, M.; Romano, P. Diversity of Saccharomyces cerevisiae strains isolated from two italian wine-producing regions. Front. Microbiol. 2016, 7, 1018. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Collins, T.S.; Masarweh, C.; Allene, G.; Heymann, H.; Ebeler, S.E.; Mills, D.A. Associations among wine grape microbiome, metabolome, and fermentation behavior suggest microbial contribution to regional wine characteristics. MBio 2016, 7, e00631. [Google Scholar] [CrossRef]
- Knight, S.; Klaere, S.; Fedrizzi, B.; Goddard, M.R. Regional microbial signatures positively correlate with differential wine phenotypes: Evidence for a microbial aspect to terroir. Sci. Rep. 2015, 5, 14233. [Google Scholar] [CrossRef]
- Santamaría, P.; López, R.; del Patrocinio Garijo, M.; Escribano, R.; González-Arenzana, L.; López-Alfaro, I.; Gutiérrez, A.R. Biodiversity of Saccharomyces cerevisiae Yeasts in Spontaneous Alcoholic Fermentations: Typical Cellar or Zone Strains? In Grape and Wine Biotechnology; Morata, A., Loira, I., Eds.; IntechOpen: London, UK, 2019; pp. 1–15. ISBN 978-953-51-2693-5. [Google Scholar] [CrossRef]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburu, F.; Querol, A. Identification of yeast by RFLP analysis of the 5.8 rRNA and the two ribosomal internal transcribed spacers. Int. J. Syst. Bacteriol. 1999, 49, 329–337. [Google Scholar] [CrossRef]
- Granchi, L.; Ganucci, D.; Viti, C.; Giovannetti, L.; Vincenzini, M. Saccharomyces cerevisiae biodiversity in spontaneous commercial fermentations of grape musts with adequate and inadequate assimilable-nitrogen content. Lett. Appl. Microbiol. 2003, 36, 54–58. [Google Scholar] [CrossRef]
- Querol, A.; Barrio, E.; Huerta, T.; Ramón, D. Molecular monitoring of wine fermentations conducted by active dry yeast strains. Appl. Environ. Microbiol. 1992, 58, 2948–2953. [Google Scholar]
- Shannon, S.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1963. [Google Scholar]
- Longo, E.; Cansado, J.; Agrelo, D.; Villa, T.G. Effect of climatic conditions on yeast diversity in grape musts from northwest Spain. Am. J. Enol. Vitic. 1991, 42, 141–144. [Google Scholar]
- Rementeria, A.; Rodriguez, J.A.; Cadaval, A.; Amenabar, R.; Muguruza, J.R.; Hernando, F.L.; Sevilla, M.J. Yeast associated with spontaneous fermentations of white wines from the “Txakoli de Bizkaia” region (Basque Country, North Spain). Int. J. Food Microbiol. 2003, 86, 201–207. [Google Scholar] [CrossRef]
- De Celis, M.; Ruiz, J.; Martín-Santamaría, M.; Alonso, A.; Marquina, D.; Navascués, E.; Gómez-Flechoso, M.A.; Belda, I.; Santos, A. Diversity of Saccharomyces cerevisiae yeasts associated to spontaneous and inoculated fermenting grapes from Spanish vineyards. Lett. Appl. Microbiol. 2019, 68, 580–588. [Google Scholar] [CrossRef]
- Tofalo, R.; Perpetuini, G.; Schirone, M.; Fasoli, G.; Aguzzi, I.; Corsetti, A. Biogeographical characterization of Saccharomyces cerevisiae wine yeast by molecular methods. Front. Microbiol. 2013, 4, 166. [Google Scholar] [CrossRef]
- Ortiz-Burgos, S. Shannon-Weaver Diversity Index. In Encyclopedia of Estuaries. Encyclopedia of Earth Sciences Series; Kennish, M.J., Ed.; Springer: Dordrecht, The Netherlands, 2016; p. 572. [Google Scholar]
- Frezier, V.; Dubourdieu, D. Ecology of yeast strains Saccharomyces cerevisiae during spontaneous fermentation in Bordeaux winery. Am. J. Enol. Vitic. 1992, 43, 375–380. [Google Scholar]
- Tofalo, R.; Perpetuini, G.; Fasoli, G.; Schirone, M.; Corsetti, A.; Suzzi, G. Biodiversity study of wine yeasts belonging to the “terroir” of Montepulciano d’Abruzzo “Colline Teramane” revealed Saccharomyces cerevisiae strains exhibiting a typical and “unique 5.8S-ITS restriction patterns”. Food Microbiol. 2014, 39, 7–12. [Google Scholar] [CrossRef]
- Schuller, D.; Cardoso, F.; Sousa, S.; Gomes, P.; Gomes, A.C.; Santos, M.A.S.; Casal, M. Genetic diversity and population structure of Saccharomyces cerevisiae strains isolated from different grape varieties and winemaking regions. PLoS ONE 2012, 7, e32507. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 2014, 111, E139–E148. [Google Scholar] [CrossRef]


| Tanks | ||||||||
|---|---|---|---|---|---|---|---|---|
| Winemaking Area | Winery Code | Grape Variety | N. | hL | Materials | SO2 (mg/L) | Temperature Control | Monitoring Time (Vintages) |
| Brunello di Montalcino (DOCG) | BM | Sangiovese | 1 | 150 | Wood | 25 | Absent | 11 |
| Val d’Orcia (Orcia DOC) | OC | Sangiovese Canaiolo Pinot noir | 6 | 50 | Wood | 30 | Present | 10 |
| Chianti Classico (DOCG) | CC1 | Sangiovese Canaiolo | 8 | 120 | Wood, steel, concrete | 28 | Absent | 4 |
| CC2 | Sangiovese | 8 | 200 | Wood | 30 | Present | 3 | |
| Winery | Vintage | N. of S. cerevisiae Isolates | Richness | H | e | Strains at Frequency >25% | Strains at Frequency 10–25% | Strains at Frequency <10% |
|---|---|---|---|---|---|---|---|---|
| BM | 1 | 23 | 10 | 1.94 | 0.84 | 1 | 1 | 8 |
| 2 | 26 | 11 | 2.10 | 0.87 | 1 | 3 | 7 | |
| 3 | 20 | 9 | 2.17 | 0.98 | 1 | 5 | 3 | |
| 4 | 21 | 13 | 2.19 | 0.85 | 1 | - | 12 | |
| 5 | 30 | 10 | 1.87 | 0.81 | 1 | 1 | 8 | |
| 6 | 20 | 8 | 1.78 | 0.85 | 2 | 2 | 4 | |
| 7 | 24 | 9 | 1.66 | 0.75 | 1 | 2 | 6 | |
| 8 | 36 | 15 | 2.11 | 0.78 | 1 | 1 | 13 | |
| 9 | 31 | 18 | 2.88 | 0.99 | - | - | 18 | |
| 10 | 37 | 9 | 1.53 | 0.70 | 2 | - | 7 | |
| 11 | 38 | 12 | 2.09 | 0.84 | 1 | 3 | 8 | |
| OD | 1 | 117 | 10 | 1.88 | 0.82 | 1 | 2 | 7 |
| 2 | 120 | 2 | 0.44 | 0.63 | 1 | 1 | - | |
| 3 | 140 | 7 | 1.03 | 0.53 | 2 | - | 5 | |
| 4 | 120 | 9 | 1.33 | 0.60 | 1 | 1 | 7 | |
| 5 | 120 | 4 | 0.41 | 0.30 | 1 | - | 3 | |
| 6 | 100 | 5 | 0.69 | 0.43 | 1 | 1 | 3 | |
| 7 | 140 | 9 | 1.87 | 0.85 | 1 | 3 | 5 | |
| 8 | 120 | 10 | 2.00 | 0.87 | 1 | 3 | 6 | |
| 9 | 140 | 9 | 1.29 | 0.59 | 1 | 2 | 6 | |
| 10 | 140 | 6 | 0.67 | 0.42 | 1 | 1 | 4 | |
| CC1 | 1 | 137 | 11 | 1.40 | 0.58 | 1 | 2 | 8 |
| 2 | 134 | 9 | 1.83 | 0.83 | 1 | 4 | 4 | |
| 3 | 126 | 4 | 0.68 | 0.49 | 1 | 1 | 2 | |
| 4 | 114 | 5 | 0.63 | 0.46 | 1 | 1 | 1 | |
| CC2 | 1 | 94 | 7 | 0.98 | 0.51 | 1 | 1 | 5 |
| 2 | 144 | 6 | 1.67 | 0.93 | 1 | 5 | - | |
| 3 | 124 | 11 | 2.05 | 0.86 | 1 | 3 | 7 |
| Winery | H | Evenness |
|---|---|---|
| BM | 2.03 ± 0.22 a | 0.84 ± 0.07 a |
| OC | 1.11 ± 0.30 b | 0.60 ± 0.09 b |
| CC1 | 1.36 ± 0.30 b | 0.59 ± 0.12 ab |
| CC2 | 1.57 ± 0.33 ab | 0.77 ± 0.10 ab |
| Strain | Vintage | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| BM-I | 8.8 | 26.9 | - | - | - | - | 50 | - | - | - | - |
| BM-II | 4.3 | - | 10 | - | - | - | - | - | - | - | - |
| BM-III | 4.3 | - | - | - | 9.9 | - | - | - | - | - | - |
| BM-IV | 8.8 | - | - | 9.4 | - | - | - | - | - | - | - |
| BM-V | - | 3.8 | 25 | - | - | - | - | 2.7 | 3.3 | 5.4 | 1.5 |
| BM-VI | - | 3.8 | 5 | 38.3 | 6.6 | 30 | 4.1 | 44.4 | 6.6 | 37.8 | 45 |
| BM-VII | - | 19.2 | 10 | - | - | - | - | - | - | - | - |
| BM-VIII | - | 3.8 | - | 4.7 | - | - | - | - | - | - | - |
| BM-IX | - | 11.5 | 15 | - | 3.3 | - | - | - | - | - | - |
| BM-X | - | - | 15 | - | - | 10 | - | - | - | 2.7 | - |
| BM-XI | - | - | - | - | - | 10 | - | 2.7 | - | - | - |
| BM-XII | - | - | - | - | - | - | - | - | 6.6 | 2.7 | - |
| BM-XIII | - | - | - | - | - | 5 | 4.1 | 13.5 | - | - | - |
| BM-XIV | - | - | - | - | - | - | 12.3 | 2.7 | - | - | - |
| BM-XV | - | - | - | - | - | - | - | 5.4 | 9.7 | 37.8 | 3 |
| BM-XVI | - | - | - | - | - | - | - | 5.4 | - | - | 8 |
| BM-XVII | - | - | - | - | - | - | - | 2.7 | 13.2 | - | 8 |
| BM-XVIII | - | - | - | - | - | - | 4.1 | 2.7 | - | - | - |
| BM-XIX | - | - | - | - | - | - | - | 2.7 | - | - | 1.5 |
| BM-XX | - | - | - | - | - | - | - | - | 3.3 | 2.7 | 8 |
| BM-XXI | - | - | - | - | - | - | - | - | - | 2.7 | 3 |
| OC-I | 0.7 | - | 1.6 | 15.7 | - | - | - | - | 22.9 | 1.9 | |
| OC-II | - | - | - | - | 0.8 | 2.9 | - | - | - | - | |
| OC-III | 16.9 | - | 1.6 | 7.8 | 3.1 | 10.3 | 3 | 9 | |||
| OC-IV | 6.3 | 16 | 27.8 | 5.5 | 5.6 | - | 5.9 | 2.8 | 12.9 | 10 | |
| OC-V | 7 | - | - | 1.2 | - | - | - | - | - | - | |
| OC-VI | 7.8 | - | 3.4 | 1.8 | - | - | - | - | - | - | |
| OC-VII | 3.1 | - | 2.5 | 6.8 | - | - | - | - | - | - | |
| OC-VIII | 14.8 | 4 | 62.3 | 60 | 90.5 | 81.7 | - | - | 0.7 | - | |
| OC-IX | 1.3 | - | - | - | - | - | 22.1 | 23 | 2.9 | 1.7 | |
| OC-X | - | - | - | 0.6 | - | - | 7.1 | - | - | - | |
| OC-XI | - | - | - | - | - | - | - | 2.4 | 31 | 20.2 | |
| OC-XII | - | - | - | - | - | - | 12.1 | 6.3 | 3.6 | 2.1 | |
| OC-XIII | - | - | - | - | - | - | 13 | - | 0.7 | 1.4 | |
| OC-XIV | - | - | - | - | - | - | - | 17.4 | 55 | 82.9 | |
| Strain | Vintage | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| CC1-I | 15 | 12.5 | 4.5 | - |
| CC1-II | 3 | - | 81 | 13 |
| CC1-III | 51.5 | 31.6 | 10 | - |
| CC1-IV | 8.8 | 1.4 | - | - |
| CC1-V | 0.8 | 2.8 | - | - |
| CC1-VI | 1.4 | 13.8 | - | 80 |
| CC1-VII | - | 16.8 | 4.5 | 7 |
| CC2-I | 71 | 12.5 | 14.5 | |
| CC2-II | 1 | 12.5 | 32 | |
| CC2-III | 1 | - | 2.5 | |
| CC2-IV | - | 12.5 | 12 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granchi, L.; Ganucci, D.; Buscioni, G.; Mangani, S.; Guerrini, S. The Biodiversity of Saccharomyces cerevisiae in Spontaneous Wine Fermentation: The Occurrence and Persistence of Winery-Strains. Fermentation 2019, 5, 86. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation5040086
Granchi L, Ganucci D, Buscioni G, Mangani S, Guerrini S. The Biodiversity of Saccharomyces cerevisiae in Spontaneous Wine Fermentation: The Occurrence and Persistence of Winery-Strains. Fermentation. 2019; 5(4):86. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation5040086
Chicago/Turabian StyleGranchi, Lisa, Donatella Ganucci, Giacomo Buscioni, Silvia Mangani, and Simona Guerrini. 2019. "The Biodiversity of Saccharomyces cerevisiae in Spontaneous Wine Fermentation: The Occurrence and Persistence of Winery-Strains" Fermentation 5, no. 4: 86. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation5040086