Growth Inhibitory and Selective Pressure Effects of Sodium Diacetate on the Spoilage Microbiota of Frankfurters Stored at 4 °C and 12 °C in Vacuum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formulation, Preparation, Storage and Sampling of Industrial Frankfurter Samples
2.2. Microbiological Analyses
2.3. Physicochemical Analyses
2.4. Sensory Evaluation
2.5. Isolation and Characterization of the Frankfurter Spoilage Microbiota During Storage
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical and Sensory Characteristics and Microbiological Quality of Fresh Frankfurters
3.2. Effect of Organic Acid Salts on Microbial Growth in VP Frankfurters during Storage at 4 °C and 12 °C
3.3. Effect of Organic Acid Salts on the pH of Spoiling Frankfurters during VP Storage at 4 °C and 12 °C
3.4. Characterization of the Spoilage LAB Isolates from VP Frankfurters Stored at 4 °C and 12 °C
3.5. Effects of the Storage Temperature on the Spoilage LAB Association of VP Frankfurters
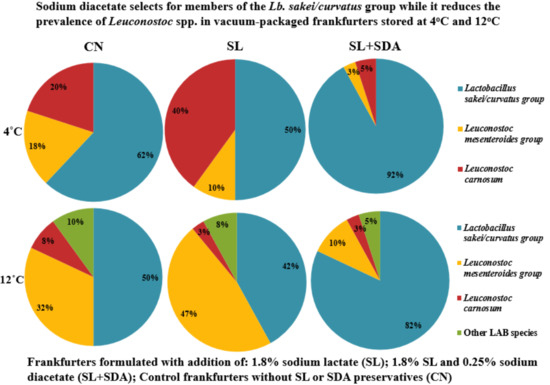
3.6. Selective Pressure Effects of SL and SL + SDA on the Spoilage LAB Association of VP Frankfurters Stored at 4 °C and 12 °C
3.7. Practical Technological Aspects for Frankfurters and Other Cured Cooked Meats
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samelis, J. Managing Microbial Spoilage in the Meat Industry. In Food Spoilage Microorganisms; de Blackburn, C.W., Ed.; CRC Woodhead Publishing Ltd.: Cambridge, UK, 2006; Chapter 9; pp. 213–286. [Google Scholar]
- Vasilopoulos, C.; De Vuyst, L.; Leroy, F. Shelf-life reduction as an emerging problem in cooked hams underlines the need for improved preservation strategies. Crit. Rev. Food Sci. Nutr. 2015, 55, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Ray, B. Prevalence and biological control of bacteriocin-producing psychrotrophic leuconostocs associated with spoilage of vacuum-packaged processed meats. J. Food Prot. 1994, 57, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, L.; Devlieghere, F.; de Graef, V.; Debevere, J. In vitro and in situ growth characteristics and behaviour of spoilage organisms associated with anaerobically stored cooked meat products. J. Appl. Microbiol. 2005, 98, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Pothakos, V.; Snauwaert, C.; De Vos, P.; Huys, G.; Devlieghere, F. Psychrotrophic members of Leuconostoc gasicomitatum, Leuconostoc gelidum and Lactococcus piscium dominate at the end of shelf-life in packaged and chilled-stored food products in Belgium. Food Microbiol. 2014, 39, 61–67. [Google Scholar] [CrossRef]
- Iulietto, M.F.; Sechi, P.; Borgogni, E.; Cenci-Goga, B.T. Meat spoilage: A critical review of a neglected alteration due to ropy slime producing bacteria. Ital. J. Anim. Sci. 2015, 14, 4011. [Google Scholar] [CrossRef]
- Blom, H.; Nerbrink, E.; Dainty, R.; Hagtvedt, T.; Borch, E.; Nissen, H.; Nesbakken, T. Addition of 2.5% lactate and 0.25% acetate controls growth of Listeria monocytogenes in vacuum-packed, sensory-acceptable servelat sausage and cooked ham at 4 °C. Int. J. Food Microbiol. 1997, 38, 71–76. [Google Scholar] [CrossRef]
- Samelis, J.; Bedie, G.K.; Sofos, J.N.; Belk, K.E.; Scanga, J.A.; Smith, G.C. Control of Listeria monocytogenes with combined antimicrobials after postprocess contamination and extended storage of frankfurters at 4 °C in vacuum packages. J. Food Prot. 2002, 65, 299–307. [Google Scholar] [CrossRef]
- Stekelenburg, F.K. Enhanced inhibition of Listeria monocytogenes in frankfurter sausage by the addition of potassium lactate and sodium diacetate mixtures. Food Microbiol. 2003, 20, 133–137. [Google Scholar] [CrossRef]
- Geornaras, I.; Skandamis, P.N.; Belk, K.E.; Scanga, J.A.; Kendall, P.A.; Smith, G.C.; Sofos, J.N. Postprocess control of Listeria monocytogenes on commercial frankfurters formulated with and without antimicrobials and stored at 10 °C. J. Food Prot. 2006, 69, 53–61. [Google Scholar] [CrossRef]
- Koo, O.K.; Eggleton, M.; O’Bryan, C.A.; Crandall, P.G.; Ricke, S.C. Antimicrobial activity of lactic acid bacteria against Listeria monocytogenes on frankfurters formulated with and without lactate/diacetate. Meat Sci. 2012, 92, 533–537. [Google Scholar] [CrossRef]
- Morey, A.; Bowers, J.W.J.; Bauermeister, L.J.; Singh, M.; Huang, T.S.; Mckee, S.R. Effect of salts of organic acids on Listeria monocytogenes, shelf-life, meat quality, and consumer acceptability of beef frankfurters. J. Food Sci. 2014, 79, M54–M60. [Google Scholar] [CrossRef] [PubMed]
- Brasileiro, I.S.; Barbosa, M.; Igarashi, M.C.; Biscola, V.; Maffei, D.F.; Landgraf, M.; Franco, B.D.G.D.M. Use of growth inhibitors for control of Listeria monocytogenes in heat-processed ready-to-eat meat products simulating post-processing contamination. LWT Food Sci. Technol. 2016, 74, 7–13. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L.L. Technologies and mechanisms for safety control of ready-to-eat muscle foods: An updated review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1886–1901. [Google Scholar] [CrossRef] [PubMed]
- Horita, C.N.; Baptista, R.C.; Caturla, M.Y.R.; Lorenzo, J.M. Combining reformulation, active packaging and non-thermal post-packaging decontamination technologies to increase the microbial quality and safety of cooked ready-to-eat meat products. Trends Food Sci. Technol. 2018, 72, 45–61. [Google Scholar] [CrossRef]
- Shelef, L.A. Antimicrobial effects of lactates: A review. J. Food Prot. 1994, 57, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Quattara, B.; Simard, R.E.; Holley, R.A.; Piete, G.J.P.; Bégin, A. Inhibitory effect of organic acids upon meat spoilage bacteria. J. Food Prot. 1997, 60, 246–253. [Google Scholar]
- Peirson, M.D.; Guan, T.Y.; Holley, R.A. Thermal resistances and lactate and diacetate sensitivities of bacteria causing bologna discolouration. Int. J. Food Microbiol. 2003, 86, 223–230. [Google Scholar] [CrossRef]
- Drosinos, E.H.; Mataragas, M.; Kampani, A.; Kritikos, D.; Metaxopoulos, I. Inhibitory effect of organic acid salts on spoilage flora in culture medium and cured cooked meat products under commercial manufacturing conditions. Meat Sci. 2006, 73, 75–81. [Google Scholar] [CrossRef]
- Benson, A.K.; David, J.R.D.; Gilbreth, S.E.; Smith, G.; Nietfeldt, J.; Legge, R.; Kim, J.; Sinha, R.; Duncan, C.E.; Ma, J.; et al. Microbial successions are associated with changes in chemical profiles of a model refrigerated fresh pork sausage during an 80-day shelf life study. Appl. Environ. Microbiol. 2014, 80, 5178–5194. [Google Scholar] [CrossRef] [Green Version]
- Bouju-Albert, A.; Pilet, M.F.; Guillou, S. Influence of lactate and acetate removal on the microbiota of French fresh pork sausages. Food Microbiol. 2018, 76, 328–336. [Google Scholar] [CrossRef]
- Collins, M.D.; Farrow, J.A.E.; Philips, B.A.; Ferusu, S.; Jones, D. Classification of Lactobacillus divergens, Lactobacillus piscicola, and some catalase-negative, asporogenous, rod-shaped bacteria from poultry in a new genus Carnobacterium. Int. J. Syst. Bacteriol. 1987, 37, 310–316. [Google Scholar] [CrossRef] [Green Version]
- Collins, M.D.; Samelis, J.; Metaxopoulos, J.; Wallbanks, S. Taxonomic studies on some leuconostoc-like organisms from fermented sausages: Description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J. Appl. Bacteriol. 1993, 75, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Doulgeraki, A.I.; Ercolini, D.; Villani, F.; Nychas, G.J.E. Spoilage microbiota associated to the storage of raw meat in different conditions. Int. J. Food Microbiol. 2012, 157, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Pothakos, V.; Devlieghere, F.; Villani, F.; Björkroth, J.; Ercolini, D. Lactic acid bacteria and their controversial role in fresh meat spoilage. Meat Sci. 2015, 109, 66–74. [Google Scholar] [CrossRef]
- Luong, N.D.M.; Corroler, L.; Zagorec, M.; Membré, J.M.; Guillou, S. Spoilage of chilled fresh meat products during storage: A quantitative analysis of literature data. Microorganisms 2020, 8, 1198. [Google Scholar] [CrossRef]
- Vasilopoulos, C.; Ravyts, F.; De Maere, H.; De Mey, E.; Paelinck, H.; De Vuyst, L.; Leroy, F. Evaluation of the spoilage lactic acid bacteria in modified-atmosphere-packaged artisan-type cooked ham using culture-dependent and culture-independent approaches. J. Appl. Microbiol. 2008, 104, 1341–1353. [Google Scholar] [CrossRef]
- Audenaert, K.; D’Haene, K.; Messens, K.; Ruyssen, T.; Vandamme, P.; Huys, G. Diversity of lactic acid bacteria from modified atmosphere packaged sliced cooked meat products at sell-by date assessed by PCR-denaturating gradient gel electrophoresis. Food Microbiol. 2010, 27, 12–18. [Google Scholar] [CrossRef]
- Geeraerts, W.; Pothakos, V.; De Vuyst, L.; Leroy, F. Diversity of the dominant bacterial species on sliced cooked pork at expiration date in the Belgian retail. Food Microbiol. 2017, 65, 236–243. [Google Scholar] [CrossRef]
- Geeraerts, W.; Pothakos, V.; De Vuyst, L.; Leroy, F. Variability within the dominant microbiota of sliced cooked poultry products at expiration date in the Belgian retail. Food Microbiol. 2018, 73, 209–215. [Google Scholar] [CrossRef]
- Vasilopoulos, C.; De Maere, H.; De Mey, E.; Paelinck, H.; De Vuyst, L.; Leroy, F. Technology-induced selection towards the spoilage microbiota of artisan-type cooked ham packed under modified atmosphere. Food Microbiol. 2010, 27, 77–84. [Google Scholar] [CrossRef]
- Comi, G.; Andyanto, D.; Manzano, M.; Iacumin, L. Lactococcus lactis and Lactobacillus sakei as bio-protective culture to eliminate Leuconostoc mesenteroides spoilage and improve the shelf life and sensorial characteristics of commercial cooked ham. Food Microbiol. 2016, 58, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Luciani, R.; Sirangelo, T.M.; Amaretti, A.; Leonardi, A.; Ulrici, A.; Foca, G.; D’Auria, G.; Moya, A.; Zuliani, V.; et al. Microbiota of sliced cooked ham packaged in modified atmosphere throughout the shelf life. Int. J. Food Microbiol. 2019, 289, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Zagdoun, M.; Coeuret, G.; N’Dione, M.; Champomier-Vergés, M.C.; Chaillou, S. Large microbiota survey reveals how the microbial ecology of cooked ham is shaped by different processing steps. Food Microbiol. 2020, 91, 103547. [Google Scholar] [CrossRef] [PubMed]
- Samelis, J.; Georgiadou, K.G. The microbial association of Greek taverna sausage stored at 4 and 10 °C in air, vacuum or 100% carbon dioxide, and its spoilage potential. J. Appl. Microbiol. 2000, 88, 58–68. [Google Scholar] [CrossRef]
- Samelis, J.; Kakouri, A. Organic acid salts in the formulation to extend the shelf life of vacuum packaged frankfurters by monitoring natural selection of post-process contaminating meat spoilage flora. In New Tools for Improving Microbial Food Safety and Quality, Proceedings of the 19th International ICFMH Symposium Food Micro 2004, Portorož, Slovenia, 12–16 September 2004; p. 124.
- Samelis, J.; Kakouri, A.; Georgiadou, K.G.; Metaxopoulos, J. Evaluation of the extent and type of bacterial contamination at different stages of processing of cooked ham. J. Appl. Microbiol. 1998, 84, 649–660. [Google Scholar] [CrossRef]
- AOAC. Meat and Meat Products. In Official Methods of Analysis, 16th ed.; 4th Rev.; Association of Official Analytic Chemists: Rockville, MD, USA, 1998; Volume II, Chapter 39. [Google Scholar]
- Samelis, J.; Kakouri, A.; Rementzis, J. Selective effect of the product type and the packaging conditions on the species of lactic acid bacteria dominating the spoilage microbial association of cooked meats at 4 °C. Food Microbiol. 2000, 17, 329–340. [Google Scholar] [CrossRef]
- Samelis, J.; Kakouri, A.; Rementzis, J. The spoilage microflora of cured, cooked turkey breasts prepared commercially with or without smoking. Int. J. Food Microbiol. 2000, 56, 133–143. [Google Scholar] [CrossRef]
- Samelis, J.; Maurogenakis, F.; Metaxopoulos, J. Characterisation of lactic acid bacteria isolated from naturally fermented Greek dry salami. Int. J. Food Microbiol. 1994, 23, 179–196. [Google Scholar] [CrossRef]
- Samelis, J.; Björkroth, J.; Kakouri, A.; Rementzis, J. Leuconostoc carnosum associated with spoilage of refrigerated whole cooked hams in Greece. J. Food Prot. 2006, 69, 2268–2273. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillacae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Torriani, S.; Van Reenen, C.A.; Klein, G.; Reuter, G.; Dellaglio, F.; Dicks, L.M.T. Lactobacillus curvatus subsp. curvatus subsp. nov. and Lactobacillus curvatus subsp. melibiosus subsp. nov. and Lactobacillus sake subsp. sake subsp. nov., new subspecies of Lactobacillus curvatus Abo- Elnaga and Kandler 1965 and Lactobacillus sake Katagiri, Kitahara, and Fukami 1934 (Klein et al. 1996, emended descriptions), respectively. Int. J. Syst. Bacteriol. 1996, 46, 1158–1163. [Google Scholar] [PubMed] [Green Version]
- Hammes, W.P.; Hertel, C. Genus I Lactobacillus Beijernick 1901, 212AL. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Whitman, W.B., Parte, A.C., Eds.; Springer: New York, NY, USA, 2009; Volume 3, pp. 465–511. [Google Scholar]
- Samelis, J.; Tsakalidou, E.; Metaxopoulos, J.; Kalantzopoulos, G. Differentiation of Lactobacillus sake and Lact. curvatus isolated from naturally fermented Greek dry salami by SDS-PAGE electrophoresis of whole-cell proteins. J. Appl. Bacteriol. 1995, 78, 157–163. [Google Scholar] [CrossRef]
- Chaillou, S.; Lucquin, I.; Najjari, A.; Zagorec, M.; Champomier-Vergès, M.C. Population genetics of Lactobacillus sakei reveals three lineages with distinct evolutionary histories. PLoS ONE 2013, 8, e73253. [Google Scholar] [CrossRef] [PubMed]
- Koort, J.; Vandamme, P.; Schillinger, U.; Holzapfel, W.; Björkroth, J. Lactobacillus curvatus subsp. melibiosus is a later synonym of Lactobacillus sakei subsp. carnosus. Int. J. Syst. Evol. Microbiol. 2004, 54, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- McLeod, A.; Nyquist, O.L.; Snipen, L.; Naterstad, K.; Axelsson, L. Diversity of Lactobacillus sakei strains investigated by phenotypic and genotypic methods. Syst. Appl. Microbiol. 2008, 31, 393–403. [Google Scholar] [CrossRef]
- Schillinger, U.; Boehringer, B.; Wallbaum, S.; Caroline, L.; Gonfa, A.; Huch, M.; Holzapfel, W.H.; Franz, C.M.A.P. A genus-specific PCR method for differentiation between Leuconostoc and Weissella and its application in identification of heterofermentative lactic acid bacteria from coffee fermentation. FEMS Microbiol. Lett. 2008, 286, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Charmpi, C.; Van Reckem, E.; Sameli, N.; Van der Veken, D.; De Vuyst, L.; Leroy, F. The use of less conventional meats or meat of high pH can lead to the growth of undesirable microorganisms during natural meat fermentation. Foods 2020, 9, 1386. [Google Scholar] [CrossRef]
- Barbieri, F.; Laghi, L.; Gardini, F.; Montanari, C.; Tabanelli, G. Metabolism of Lactobacillus sakei Chr82 in the presence of different amounts of fermentable sugars. Foods 2020, 9, 720. [Google Scholar] [CrossRef]
- Korkeala, H.; Suortti, T.; Makela, P. Ropy slime formation in vacuum-packed cooked meat products caused by homofermentative lactobacilli and a Leuconostoc species. Int. J. Food Microbiol. 1988, 7, 339–347. [Google Scholar] [CrossRef]
- Cenci-Goga, B.T.; Sechi, P.; Iulietto, M.F.; Amirjalali, S.; Barbera, S.; Karama, M.; Aly, S.S.; Grispoldi, L. Characterization and growth under different storage temperatures of ropy slime-producing Leuconostoc mesenteroides isolated from cooked meat products. J. Food Prot. 2020, 83, 1043–1049. [Google Scholar] [CrossRef]
- Andreevskaya, M.; Jääskeläinen, E.; Johansson, P.; Ylinen, A.; Paulin, L.; Björkroth, J.; Auvinen, P. Food spoilage-associated Leuconostoc, Lactococcus, and Lactobacillus species display different survival strategies in response to competition. Appl. Environ. Microbiol. 2018, 84, e00554-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieminen, T.T.; Nummela, M.; Björkroth, J. Packaging gas selects lactic acid bacterial communities on raw pork. J. Appl. Microbiol. 2015, 119, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Iacumin, L.; Cappellari, G.; Colautti, A.; Comi, G. Listeria monocytogenes survey in cubed cooked ham packaged in modified atmosphere and bioprotective effects of selected lactic acid bacteria. Microorganisms 2020, 8, 898. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, L.; Devlieghere, F.; Debevere, J. Evaluation of meat born lactic acid bacteria as protective cultures for the biopreservation of cooked meat products. Int. J. Food Microbiol. 2004, 96, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulos, C.; De Mey, E.; Dewulf, L.; Paelinck, H.; De Smedt, A.; Vandendriessche, F.; De Vuyst, L.; Leroy, F. Interactions between bacterial isolates from modified-atmosphere-packaged artisan-type cooked ham in view of the development of a bioprotective culture. Food Microbiol. 2010, 27, 1086–1094. [Google Scholar] [CrossRef]



| Storage Temperature | Frankfurter Treatment a | pH Value of Frankfurter Samples During Storage (Days) b | |||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 15 | 30 | 60 | 90 | ||
| 4 °C | CN | 6.52 C b (0.05) | 6.47 BC b (0.03) | 6.46 BC b (0.03) | 6.41 B c (0.03) | 5.74 A bc (0.05) | 5.67 A b (0.12) |
| SL | 6.40 B b (0.14) | 6.39 B b (0.02) | 6.39 B b (0.04) | 6.44 B c (0.04) | 6.32 B e (0.11) | 6.15 A c (0.15) | |
| SL + SDA | 6.05 A a (0.10) | 6.12 AB a (0.16) | 6.21 B a (0.05) | 6.15 AB b (0.02) | 6.17 AB d (0.06) | 6.16 AB c (0.03) | |
| 12 °C | CN | 6.52 E b (0.05) | 6.42 DE b (0.08) | 6.22 D a (0.09) | 5.75 C a (0.22) | 5.41 B a (0.12) | 5.07 A a (0.24) |
| SL | 6.40 D b (0.14) | 6.40 D b (0.02) | 6.40 D b (0.03) | 6.00 C b (0.04) | 5.78 B c (0.03) | 5.16 A a (0.27) | |
| SL + SDA | 6.05 B a (0.10) | 6.26 B a (0.19) | 6.19 B a (0.09) | 6.04 B b (0.11) | 5.60 A b (0.17) | 5.43 A b (0.02) | |
| LAB Species Identified | Lactobacillus sakei | Lactobacillus curvatus | Unidentified lactobacillus | Lactobacillus plantarum | Leuconostoc mesenteroides | Leuconostoc carnosum | Weissella paramesenteroides | ||
|---|---|---|---|---|---|---|---|---|---|
| Group/subgroup-biotype | A1 | A2 | A3 | B | C | D | E | F | G |
| Isolated from TSAYE | 25 | 3 | 6 | 38 | 1 | 5 | 21 | 15 | 6 |
| Isolated from MRS | 26 | 4 | 3 | 47 | 2 | 1 | 16 | 16 | 5 |
| No. of LAB isolates | 51 | 7 | 9 | 85 | 3 | 6 | 37 | 31 | 11 |
| Cell morphology | R | R | R | R | R | R | CB | CB | CB |
| CO2 from glucose | - | - | - | - | - | - | + | + | + |
| ΝH3 from arginine | + | + | - | - | + | - | - | - | - |
| Growth at 37 °C | + | + | + | + | + | + | + | - | + |
| Slime from sucrose | - | - | - | - | + | - | ++ | 10/31 | - |
| Fermentation of | |||||||||
| L-arabinose | 31/51 | - | 3/9 | - | + | + | + | - | + |
| Cellobiose | 30/51 | - | +/+d | 65/85 | + | + | 8/37 | 16/31 | +/+d |
| Galactose | + | + | + | + | + | + | + | - | + |
| Lactose | 22/51 | - | 3/9 | 49/85 | + | + | +/+d | - | +/+d |
| Maltose | 6/51 | - | 6/9 | 81/85 | + | + | + | 16/31 | + |
| Mannitol | - | - | - | - | - | + | 27/37 | - | +/(+)d |
| Melibiose | + | - | + | - | + | + | + | - | + |
| Raffinose | - | - | - | - | + | + | +/+d | - | +/(+)d |
| Ribose | + | + | + | + | + | + | + | 9/31 | + |
| Sorbitol | - | - | - | - | - | + | NT | NT | NT |
| Sucrose | + | + | 5/9 | - | + | + | + | + | + |
| Trehalose | + | + | + | 16/85 | + | + | + | 20/31 | + |
| Xylose | - | - | - | - | + | - | + | - | 8/11 |
| LAB Species/Biotype (Group in Table 2) | Number of Isolates (No) | Distribution of Isolates (% Total No) | Isolates from Frankfurter Samples at 4 °C b | Isolates from Frankfurter samples at 12 °C b | Relative Distribution of the Isolates within each Frankfurter Treatment b | ||
|---|---|---|---|---|---|---|---|
| CN | SL | SL + SDA | |||||
| Lactobacillus sakei (Group A/A1) | 51 | 21.3 | 26 (21.7) | 25 (20.8) | 22 (27.5) | 14 (17.5) | 15 (18.7) |
| Lactobacillus sakei/curvatus (A2) | 7 | 2.9 | 7 (5.8) | 0 (0.0) | 3 (3.8) | 1 (1.2) | 3 (3.8) |
| Lactobacillus curvatus/’melibiosus’ (A3) | 9 | 3.8 | 1 (0.8) | 8 (6.7) | 0 (0.0) | 1 (1.2) | 8 (10.0) |
| Lactobacillus curvatus (Group B) | 85 | 35.4 | 48 (40.0) | 37 (30.8) | 20 (25.0) | 21 (26.3) | 44 (55.0) |
| Lactobacillus sakei/curvatus taxon | 152 | 63.4 | 82 (68.3) | 70 (58.3) | 45 (56.3) | 37 (46.2) | 70 (87.5) |
| Unidentified Lactobacillus (Group C) | 3 | 1.3 | 0 (0.0) | 3 (2.5) | 1 (1.2) | 2 (2.5) | 0 (0.0) |
| Lactobacillus plantarum (Group D) | 6 | 2.5 | 0 (0.0) | 6 (5.0) | 3 (3.8) | 1 (1.2) | 2 (2.5) |
| Additional facultative heterofermentative Lactobacillus spp. | 9 | 3.8 | 0 (0.0) | 9 (7.5) | 4 (5.0) | 3 (3.7) | 2 (2.5) |
| Leuconostoc mesenteroides (Group E) | 37 | 15.4 | 7 (5.8) | 30 (25.0) | 12 (15.0) | 21 (26.3) | 4 (5.0) |
| Leuconostoc carnosum (Group F) | 31 | 12.9 | 26 (21.7) | 5 (4.2) | 11 (13.7) | 17 (21.3) | 3 (3.8) |
| Weissella paramesenteroides (Group G) | 11 | 4.5 | 5 (4.2) | 6 (5.0) | 8 (10.0) | 2 (2.5) | 1 (1.2) |
| Obligatory heterofermentative LAB | 79 | 32.8 | 38 (31.7) | 41 (34.2) | 31 (38.7) | 40 (50.1) | 8 (10.0) |
| Total LAB isolates | 240 | 100 | 120 | 120 | 80 | 80 | 80 |
| LAB Species Identified | Storage at 4 °C | Storage at 12 °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Group/Subgroup in Table 2) | CN | SL | SL + SDA | CN | SL | SL + SDA | ||||||
| Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | |
| Lactobacillus sakei (Group A/A1) | 5 | 1 | 4 | - | 3 | - | 4 | 1 | 3 | - | 4 | - |
| Lactobacillus sakei/curvatus (Group A/A2) | 1 | - | 1 | - | 1 | - | - | - | - | - | - | - |
| Lactobacillus curvatus/ ‘melibiosus’ (Group A/A3) | - | - | - | - | 1 | - | - | - | - | 1 | 1 | 3 |
| Lactobacillus curvatus (Group B) | 1 | 4 | - | 5 | 5 | 9 | - | 3 | - | 3 | 4 | 4 |
| Unidentified Lactobacillus (Group C) | - | - | - | - | - | - | - | 1 | - | - | - | - |
| Lactobacillus plantarum (Group D) | - | - | - | - | - | - | 1 | 2 | - | 1 | - | 1 |
| Leuconostoc mesenteroides (Group E) | 1 | 2 | 1 | 2 | - | - | 2 | 1 | 6 | 4 | 1 | 1 |
| Leuconostoc carnosum (Group F) | 2 | 2 | 4 | 2 | - | - | 1 | 2 | - | 1 | - | 1 |
| Weissella paramesenteroides (Group G) | - | 1 | - | 1 | - | 1 | 2 | - | 1 | - | - | - |
| LAB Species | Storage at 4 °C | Storage at 12 °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Group/Subgroup in Table 2) | CN | SL | SL + SDA | CN | SL | SL + SDA | ||||||
| Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | |
| Lactobacillus sakei (Group A/A1) | 4 | 3 | 2 | 1 | 3 | - | 4 | - | 4 | - | 5 | - |
| Lactobacillus sakei/curvatus (Group A/A2) | 2 | - | - | - | 2 | - | - | - | - | - | - | - |
| Lactobacillus curvatus/ ‘melibiosus’ (Group A/A3) | - | - | - | - | - | - | - | - | - | - | 2 | 1 |
| Lactobacillus curvatus (Group B) | - | 4 | 2 | 5 | 4 | 9 | 3 | 5 | 2 | 4 | - | 9 |
| Unidentified Lactobacillus (Group C) | - | - | - | - | - | - | - | - | - | 2 | - | - |
| Lactobacillus plantarum (Group D) | - | - | - | - | - | - | - | - | - | - | 1 | - |
| Leuconostoc mesenteroides (Group E) | - | 1 | - | - | - | - | 2 | 3 | 4 | 4 | 2 | - |
| Leuconostoc carnosum (Group F) | 3 | 1 | 6 | 4 | 1 | 1 | - | - | - | - | - | - |
| Weissella paramesenteroides (Group G) | 1 | 1 | - | - | - | - | 1 | 2 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samelis, J.; Kakouri, A. Growth Inhibitory and Selective Pressure Effects of Sodium Diacetate on the Spoilage Microbiota of Frankfurters Stored at 4 °C and 12 °C in Vacuum. Foods 2021, 10, 74. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10010074
Samelis J, Kakouri A. Growth Inhibitory and Selective Pressure Effects of Sodium Diacetate on the Spoilage Microbiota of Frankfurters Stored at 4 °C and 12 °C in Vacuum. Foods. 2021; 10(1):74. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10010074
Chicago/Turabian StyleSamelis, John, and Athanasia Kakouri. 2021. "Growth Inhibitory and Selective Pressure Effects of Sodium Diacetate on the Spoilage Microbiota of Frankfurters Stored at 4 °C and 12 °C in Vacuum" Foods 10, no. 1: 74. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10010074