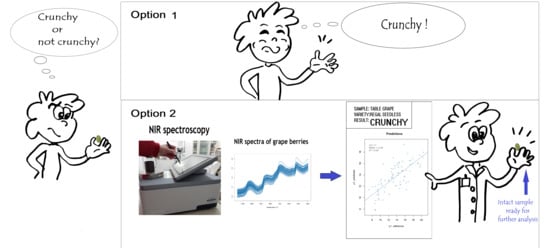
NIR Analysis of Intact Grape Berries: Chemical and Physical Properties Prediction Using Multivariate Analysis
Abstract
:1. Introduction
1.1. Grape Texture Attributes
1.2. NIR Spectroscopy
1.3. Prediction Models
2. Material and Methods
2.1. Grape Barriers Samples
2.2. NIR Spectra Measurements
2.3. Instrumental Texture Analysis
2.4. Soluble Solids Measurements
2.5. Statistical Analysis
3. Results and Discussion
3.1. NIR Spectra Pretreatments
3.2. NIR Spectra Analysis: Principal Component Analysis
3.3. Texture Data
3.3.1. Multivariate Calibration and Validation on “Hardness” Data
3.3.2. iPLS on “Hardness” Data
3.4. TSS Data
3.4.1. Multivariate Calibration and Validation on TSS Data
3.4.2. iPLS on TSS Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kohyama, K. Chapter 1. Food Texture—Sensory Evaluation and Instrumental Measurement. In Textural Characteristics of World Foods; Nishinari, K., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 1–13. [Google Scholar] [CrossRef]
- Rolle, L.; Siret, R.; Río Segade, S.; Maury, C.; Gerbi, V.; Jourjon, F. Instrumental texture analysis parameters as markers of table-grape and winegrape quality: A review. Am. J. Enol. Vitic. 2012, 63, 11–28. [Google Scholar] [CrossRef] [Green Version]
- Yakushiji, H.; Sakurai, N.; Morinaga, K. Changes in cell-wall polysaccharides from the mesocarp of grape berries during veraison. Physiol. Plant 2008, 111, 188–195. [Google Scholar] [CrossRef]
- Giacosa, S.; Zeppa, G.; Baiano, A.; Torchio, F.; Río Segade, S.; Gerbi, V.; Rolle, L. Assessment of sensory firmness and crunchiness of table grapes by acoustic and mechanical properties. Aust. J. Grape Wine Res. 2015, 21, 213–225. [Google Scholar] [CrossRef]
- Antonacci, D.; Genghi, R.; Perniola, R.; Alba, V.; Roccotelli, S. Densità di impianto e qualità dell’uva Regal Seedless: Selezionato un clone più produttivo. Riv. Fruttic. Ortofloric. Ed. Agric. 2015, 77, 15–18. [Google Scholar]
- Bart, J.C.J.; Gucciardi, E.; Cavallaro, S. Chapter 8—Quality assurance of biolubricants. In Woodhead Publishing Series in Energy, Biolubricants; Bart, J.C.J., Gucciardi, E., Cavallaro, S., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 396–450. ISBN 9780857092632. [Google Scholar] [CrossRef]
- Chung, H. Applications of Near-Infrared Spectroscopy in Refineries and Important Issues to Address. Appl. Spectrosc. Rev. 2007, 42, 251–285. [Google Scholar] [CrossRef]
- Beghi, R.; Buratti, S.; Giovenzana, V.; Benedetti, S.; Guidetti, R. Electronic nose and visible-near infrared spectroscopy in fruit and vegetable monitoring. Rev. Anal. Chem. 2017, 36. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: http://www.R-project.org/ (accessed on 1 November 2020).
- Rolle, L.; Río Segade, S.; Torchio, F.; Giacosa, S.; Cagnasso, E.; Marengo, F.; Gerbi, V. Influence of grape density and harvest date on changes in phenolic composition, phenol extractability indices, and instrumental texture properties during ripening. J. Agric. Food Chem. 2011, 59, 8796–8805. [Google Scholar] [CrossRef]
- Rolle, L.; Giacosa, S.; Gerbi, V.; Novello, V. Comparative study of texture properties, color characteristics, and chemical composition of ten white table-grape varieties. Am. J. Enol. Vitic. 2011, 62, 49–56. [Google Scholar] [CrossRef]
- Kucheryavskiy, S. mdatools—R package for chemometrics. Chemom. Intell. Lab. Syst. 2020, 198, 103937. [Google Scholar] [CrossRef]
- Signal Developers, Signal: Signal Processing. 2013. Available online: http://r-forge.r-project.org/projects/signal/ (accessed on 1 November 2020).
- Vu, V.Q. ggbiplot: A ggplot2 Based Biplot. R Package, Version 0.55. 2011. Available online: http://github.com/vqv/ggbiplot (accessed on 1 November 2020).
- Coombes, K.R.; Fritsche, H.A., Jr.; Clarke, C.; Chen, J.; Baggerly, K.A.; Morris, J.S.; Xiao, L.; Hung, M.; Kuerer, H.M. Quality control and peak finding for proteomics data collected from nipple aspirate fluid by surface-enhanced laser desorption and ionization. Clin. Chem. 2003, 49, 1615–1623. [Google Scholar] [CrossRef]
- Azzouz, T.; Puigdoménech, A.; Aragay, M.; Tauler, R. Comparison between different data pretreatment methods in the analysis of forage samples using near-infrared diffuse reflectance spectroscopy and partial least-squares multivariate calibration method. Anal. Chim. Acta 2003, 484, 121–134. [Google Scholar] [CrossRef]
- Sabatier, D.; Dardenne, P.; Thuriès, L. Near Infrared Reflectance Calibration Optimisation to Predict Lignocellulosic Compounds in Sugarcane Samples with Coarse Particle Size. J. Near Infrared Spectros. 2011, 19, 199–209. [Google Scholar] [CrossRef]
- Awotwe-Otoo, D.; Zidanm, A.; Rahman, Z.; Habib, M.J. Evaluation of Anticancer Drug-Loaded Nanoparticle Characteristics by Nondestructive Methodologies. AAPS PharmSciTech 2012, 13, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basile, T.; Marsico, A.D.; Cardone, M.F.; Antonacci, D.; Perniola, R. FT-NIR Analysis of Intact Table Grape Berries to Understand Consumer Preference Driving Factors. Foods 2020, 9, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bampi, M.; Scheer, A.D.P.; de Castilhos, F. Application of near infrared spectroscopy to predict the average droplet size and water content in biodiesel emulsions. Fuel 2013, 113, 546–552. [Google Scholar] [CrossRef] [Green Version]
- Rinnan, Å.; van den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Fernández-Novales, J.; Tardáguila, J.; Gutiérrez, S.; Diago, M.P. On-The-Go VIS + SW − NIR Spectroscopy as a Reliable Monitoring Tool for Grape Composition within the Vineyard. Molecules 2019, 24, 2795. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, P.; Soares, A.; Castanho, A.; Almeida, A.S.; Oliveira, J.; Brites, C. Dataset of Near-infrared spectroscopy measurement for amylose determination using PLS algorithms. Data Brief 2017, 15, 389–396. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Xie, S.F.; Xiang, B.R.; Yu, L.Y.; Deng, H.S. Tailoring noise frequency spectrum to improve NIR determinations. Talanta 2009, 80, 895–902. [Google Scholar] [CrossRef]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Trygg, J.; Wikström, C.; Wold, S. Multi- and Megavariate Data Analysis. Part I: Basic Principles and Applications, 2nd ed.; UMETRICS AB: Umeå, Sweden, 2006. [Google Scholar]
- Marsico, A.D.; Perniola, R.; Cardone, M.F.; Velenosi, M.; Antonacci, D.; Alba, V.; Basile, T. Study of the Influence of Different Yeast Strains on Red Wine Fermentation with FT-NIR Spectroscopy and Principal Component Analysis. J. Multidiscip. Sci. J. 2018, 1, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Acri, G.; Testagrossa, B.; Vermiglio, G. FT-NIR Analysis of Different Garlic Cultivars. J. Food Meas. Charact. 2016, 10, 127–136. [Google Scholar] [CrossRef]
- Saha, P.; Roy, N.; Mukherjee, D.; Kumar Sarkar, A. Application of principal component analysis for outlier detection in heterogeneous traffic data. Procedia Comput. Sci. 2016, 83, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Rodionova, O.Y.; Pomerantsev, A.L. Detection of Outliers in Projection-Based Modeling. Anal. Chem. 2020, 92, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Pomerantsev, A.L.; Rodionova, O.Y. Concept and role of extreme objects in PCA/SIMCA. J. Chemom. 2014, 28, 429–438. [Google Scholar] [CrossRef]
- Pomerantsev, A.L. Acceptance areas for multivariate classification derived by projection methods. J. Chemom. 2008, 22, 601–609. [Google Scholar] [CrossRef]
- Rasti, B.; Scheunders, P.; Ghamisi, P.; Licciardi, G.; Chanussot, J. Noise reduction in hyperspectral imagery: Overview and application. Remote Sens. 2018, 3, 482. [Google Scholar] [CrossRef] [Green Version]
- Conzen, J.P. Multivariate Calibration, 3rd ed.; Bruker Optik GmbH: Ettlingen, Germany, 2014; ISBN 978-3-929431-13-1. [Google Scholar]
- Chang, C.-W.; Laird, D.A.; Mausbach, M.J.; Hurburgh, C.R., Jr. Near-Infrared reflectance spectroscopy-principal components regression analyses of soil properties. Soil Sci. Soc. Am. J. 2001, 65, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.C.; Sobering, D.C. Comparison of commercial near infrared transmittance and reflectance instruments for analysis of whole grains and seeds. J. Near Infrared Spectrosc. 1993, 1, 25–32. [Google Scholar] [CrossRef]
- Bellon-Maurel, V.; Fernandez-Ahumada, E.; Palagos, B.; Roger, J.M.; Mc Bratney, A. Critical review of chemometric indicators commonly used for assessing the quality of the prediction of soil attributes by NIR spectroscopy. TrAC Trends Anal. Chem. 2010, 29, 1073–1081. [Google Scholar] [CrossRef]
- Mehmood, T.; Hovde Liland, K.; Snipen, L.; Sæbø, S. A review of variable selection methods in Partial Least Squares Regression. Chemom. Intell. Lab. Syst. 2012, 118, 62–69. [Google Scholar] [CrossRef]
- Nørgaard, L.; Saudland, A.; Wagner, J.; Nielser, J.P.; Munck, L.; Engelsen, S.B. Interval Partial Least-Squares Regression (iPLS): A Comparative Chemometric Study with an Example from Near-Infrared Spectroscopy. Appl. Spectrosc. 2000, 54, 413–419. [Google Scholar] [CrossRef]
- ISO 21748:2017(EN). Guidance for The Use of Repeatability, Reproducibility and Trueness Estimates in Measurement Uncertainty Evaluation. Available online: https://www.iso.org/standard/71615.html (accessed on 4 December 2020).
- Cozzolino, D.; Cynkar, W.; Shah, N.; Smith, P. Quantitative analysis of minerals and electric conductivity of red grape homogenates by near infrared reflectance spectroscopy. Comput. Electron. Agric. 2011, 77, 81–85. [Google Scholar] [CrossRef]
- de Oliveira, G.A.; Bureau, S.; Renard, C.M.G.C.; Pereira-Netto, A.B.; de Castilhos, F. Comparison of NIRS approach for prediction of internal quality traits in three fruit species. Food Chem. 2014, 143, 223–230. [Google Scholar] [CrossRef]


















| Pretreatment | Parameters | PC1% | PC2% |
|---|---|---|---|
| MSC | - | 51.6 | 27.8 |
| SNV | - | 51.6 | 27.9 |
| SGD1 | 1 31 1 | 28.8 | 15.2 |
| SGD2 | 3 31 2 | 6.3 | 5 |
| MSC+SG smoothing | - | 78.5 | 9.1 |
| MSC+SGD1 | 1 31 1 | 25.1 | 14.8 |
| MSC+SGD2 | 3 31 2 | 5.6 | 4.3 |
| Data Set | Model | X Cumexpvar | Y Cumexpvar | R2 | RMSE | Slope | Bias | RPD |
|---|---|---|---|---|---|---|---|---|
| Training set (all spectra) nComp = 4 | Cal | 88.88688 | 50.56211 | 0.506 | 2.312 | 0.506 | 0.000 | 1.43 |
| Cv | NA | NA | 0.305 | 2.741 | 0.383 | −0.0005 | 1.21 | |
| PCA outlier removal nComp = 4 | Cal | 84.40549 | 55.65603 | 0.557 | 2.185 | 0.557 | 0.0000 | 1.51 |
| Cv | NA | NA | 0.327 | 2.692 | 0.423 | −0.0468 | 1.23 | |
| Extreme values removal nComp = 4 | Cal | 86.99243 | 53.71146 | 0.537 | 2.181 | 0.537 | 0.000 | 1.48 |
| Cv | NA | NA | 0.325 | 2.633 | 0.411 | 0.028 | 1.22 |
| Data Set | Model | X Cumexpvar | Y Cumexpvar | R2 | RMSE | Slope | Bias | RPD |
|---|---|---|---|---|---|---|---|---|
| All training set nComp = 4 | initial global model | 98.47121 | 47.09408 | 0.471 | 2.392 | 0.471 | 0.0000 | 1.38 |
| final model | NA | NA | 0.405 | 2.536 | 0.440 | 0.0176 | 1.30 | |
| PCA outlier removal nComp = 3 | initial global model | 76.11868 | 43.80306 | 0.438 | 2.460 | 0.438 | 0.0000 | 1.34 |
| final model | NA | NA | 0.391 | 2.561 | 0.402 | −0.0120 | 1.29 | |
| Extreme values removal nComp = 3 | initial global model | 86.49316 | 40.5588 | 0.406 | 2.471 | 0.406 | 0.0000 | 1.31 |
| final model | NA | NA | 0.376 | 2.531 | 0.394 | 0.0412 | 1.27 |
| Data Set | Model | X Cumexpvar | Y Cumexpvar | R2 | RMSE | Slope | Bias | RPD |
|---|---|---|---|---|---|---|---|---|
| Training set (all spectra)nComp = 6 | Cal | 91.90354 | 83.1635 | 0.832 | 0.663 | 0.832 | 0.0000 | 2.45 |
| Cv | NA | NA | 0.611 | 1.008 | 0.621 | 0.0158 | 1.61 | |
| PCA outlier removal nComp = 7 | Cal | 91.34024 | 94.56569 | 0.946 | 0.371 | 0.946 | 0.0000 | 4.32 |
| Cv | NA | NA | 0.619 | 0.982 | 0.598 | 0.0415 | 1.63 |
| Data Set | Model | X Cumexpvar | Y Cumexpvar | R2 | RMSE | Slope | Bias | RPD |
|---|---|---|---|---|---|---|---|---|
| Training set (all spectra) nComp = 4 | Cal | 95.33922 | 82.98385 | 0.830 | 0.667 | 0.830 | 0.0000 | 2.44 |
| Cv | NA | NA | 0.659 | 0.943 | 0.615 | 0.0145 | 1.72 | |
| PCA outlier removal nComp = 5 | Cal | 95.69764 | 87.0354 | 0.870 | 0.573 | 0.870 | 0.0000 | 2.79 |
| Cv | NA | NA | 0.701 | 0.870 | 0.655 | 0.0191 | 1.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basile, T.; Marsico, A.D.; Perniola, R. NIR Analysis of Intact Grape Berries: Chemical and Physical Properties Prediction Using Multivariate Analysis. Foods 2021, 10, 113. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10010113
Basile T, Marsico AD, Perniola R. NIR Analysis of Intact Grape Berries: Chemical and Physical Properties Prediction Using Multivariate Analysis. Foods. 2021; 10(1):113. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10010113
Chicago/Turabian StyleBasile, Teodora, Antonio Domenico Marsico, and Rocco Perniola. 2021. "NIR Analysis of Intact Grape Berries: Chemical and Physical Properties Prediction Using Multivariate Analysis" Foods 10, no. 1: 113. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10010113