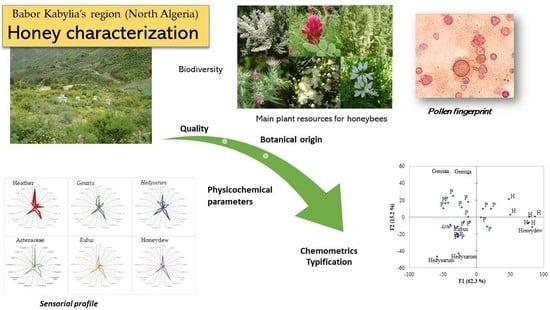
Sensorial, Melissopalynological and Physico-Chemical Characteristics of Honey from Babors Kabylia’s Region (Algeria)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Honey Samples
2.2. Sensorial Analysis
2.3. Melissopalynological Analysis
2.3.1. Quantitative
2.3.2. Qualitative
2.4. Physico-Chemical Analysis and Color Determination
2.4.1. Honey Freshness
2.4.2. Other Quality Parameters
2.4.3. Color of Honey
2.4.4. Polyphenol and Flavonoid Content
2.4.5. Mineral Composition Analysis
2.5. Data Analysis
3. Results and Discussion
3.1. Sensorial Profile of Samples
3.2. Pollen Spectra and Main Botanical Origin of Samples
3.3. Quality Evaluation of the Honey Samples
3.3.1. Mineral Content
3.3.2. Total Phenolic and Flavonoid Content
3.4. Multivariate Analysis Applied to the Interpretation of Samples Characteristics and Typification of the Honey Samples
3.5. Characteristics of the Different Honey Types
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hussein, M.H. A review of beekeeping in Arab countries. Bee World 2000, 81, 56–71. [Google Scholar] [CrossRef]
- Boutabia, L.; Telailia, S.; Chefrour, A. Spectre pollinique de miels d’abeille (Apis mellifera L.) de la région d’El Tarf (Nord-Est algérien). Livest. Res. Rural. Dev. 2016, 28, 1–8. [Google Scholar]
- Véla, E.; Benhouhou, S. Évaluation d’un nouveau point chaud de biodiversité végétale dans le Bassin méditerranéen (Afrique du Nord). Comptes Rendus Biol. 2007, 330, 589–605. [Google Scholar] [CrossRef]
- Ouchemoukh, S.; Louaileche, H.; Schweitzer, P. Physicochemical characteristics and pollen spectrum of some Algerian honeys. Food Control 2007, 18, 52–58. [Google Scholar] [CrossRef]
- Haderbache, L.; Mouna, B.; Arezki, M. Ziziphus lotus and Euphorbia bupleuroides Algerian honeys. World Appl. Sci. J. 2013, 24, 1536–1543. [Google Scholar]
- Otmani, I.; Abdennour, C.; Dridi, A.; Kahalerras, L.; Salem, A.H. Characteristics of the bitter and sweet honey from Algeria Mediterranean coast. Vet. World 2019, 12, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Homrani, M.; Escuredo, O.; Rodríguez-Flores, M.S.; Fatiha, D.; Mohammed, B.; Homrani, A.; Seijo, M.C. Botanical Origin, Pollen Profile, and Physicochemical Properties of Algerian Honey from Different Bioclimatic Areas. Foods 2020, 9, 938. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, M.; Tarapatskyy, M.; Dżugan, M. The influence of geographical origin on honey composition studied by Polish and Slovak honeys. Cze. J. Food Sci. 2019, 37, 232–238. [Google Scholar] [CrossRef]
- di Marco, G.; Manfredini, A.; Leonardi, D.; Canuti, L.; Impei, S.; Gismondi, A.; Canini, A. Geographical, botanical and chemical profile of monofloral Italian honeys as food quality guarantee and territory brand. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2017, 151, 450–463. [Google Scholar] [CrossRef]
- Gharzouli, R. Flore et Végétation de la Kabylie des Babors Etude Floristique et Phytosociologique des Groupements Forestiers et Post-Forestier des Djbels Takoucht, Adrar Ou-Mellal, Tababort et Babor. Ph.D. Thesis, Ferhat Abbas University, Sétif, Algeria, 2007. [Google Scholar]
- Rana, S.; Mishra, M.; Yadav, D.; Subramani, S.K.; Katare, C.; Prasad, G. Medicinal uses of honey: A review on its benefits to human health. Prog. Nutr. 2018, 20, 5–14. [Google Scholar]
- Marcazzan, G.L.; Caretta, C.M.; Marchese, C.M.; Piana, M.L. A review of methods for honey sensory analysis. J. Apic. Res. 2018, 57, 75–87. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Bogdanov, S.; Martin, P.; Lüllmann, C. Harmonized methods of the European honey commission. International Honey Commission. Apidologie 1997, 1–59. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Grand, A.A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar]
- Caroli, S.; Forte, G.; Iamiceli, A.L.; Galoppi, B. Determination of essential and potentially toxic trace elements in honey by inductively coupled plasma-based techniques. Talanta 1999, 50, 327–336. [Google Scholar] [CrossRef]
- The Council of the European Union. Council Directive 2001/110/EC of 20 December 2001 relating to honey. Off. J. Eur. Communities 2002, L10, 47–52. [Google Scholar]
- Karabagias, I.K.; Badeka, A.V.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Botanical discrimination of Greek unifloral honeys with physico-chemical and chemometric analyses. Food Chem. 2014, 165, 181–190. [Google Scholar] [CrossRef]
- Escuredo, O.; Míguez, M.; González, M.F.; Seijo, M.C. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013, 138, 851–856. [Google Scholar] [CrossRef]
- Bilandžić, N.; Gajger, I.T.; Kosanovi’c, M.; Calopek, B.; Sedak, M.; Kolanovi´c, B.S.; Varenina, I.; Luburi’c, D.B.; Varga, I.; Đoki’c, M. Essential and toxic element concentrations in monofloral honeys from southern Croatia. Food Chem. 2017, 234, 245–253. [Google Scholar] [CrossRef]
- Tafinine, Z.M.; Ouchemoukh, S.; Tamendjari, A. Antioxidant activity of some Algerian honey and propolis. Ind. Crops Prod. 2016, 88, 85–90. [Google Scholar] [CrossRef]
- Dahmani, K.; Houdeib, J.B.; Zouambi, A.; Bendeddouche, B.; Fernández-Muiño, M.; Osés, S.M.; Sancho, M.T. Quality Attributes of Local and Imported Honeys Commercialized in Algeria. J. Apic. Sci. 2020, 1, 251–262. [Google Scholar] [CrossRef]
- di Marco, G.; Gismondi, A.; Panzanella, L.; Canuti, L.; Impei, S.; Leonardi, D.; Canini, A. Botanical influence on phenolic profile and antioxidant level of Italian honeys. J. Food Sci. Technol. 2018, 55, 4042–4050. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Abrini, J.; Touys, A.E.; Lagrouh, F.; Dakka, N.; Bakri, Y. Analyse phytochimique et évaluation de l’activité antioxydante des échantillons du miel marocain. Phytothérapie 2018, 16, S220–S224. [Google Scholar] [CrossRef]
- Lachman, J.; Hejtmankova, A.; Sýkora, J.; Karban, J.; Orsak, M.; Rygerova, B. Contents of major phenolic and flavonoid antioxidants in selected Czech honey. Czech. J. Food Sci. 2010, 28, 412–426. [Google Scholar] [CrossRef] [Green Version]
- Dong, R.; Zheng, Y.N.; Xu, B.J. Phenolic Profiles and Antioxidant Capacities of Chinese Unifloral Honeys from Different Botanical and Geographical Sources. Food Bioprocess Technol. 2013, 6, 762–770. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Haouam, L.; Tahar, A.; Dailly, H.; Lahrichi, A.; Chaqroune, A.; Abdennour, C. Physicochemical properties and major elements contents of Algerian honeys from semi-arid regions. Emir. J. Food Agric. 2016, 28, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Tafinine, Z.M.; Ouchemoukh, S.; Bachir Bey, M.; Louaileche, H.; Tamendjari, A. Effect of storage on hydroxymethylfurfural (HMF) and color of some Algerian honey. Int. Food Res. J. 2018, 25, 1044–1050. [Google Scholar]
- Haouam, L.; Dailly, H.; Bruneau, E.; Tahar, A. The quality of honeys influenced by the traditional heating method. J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1276–1280. [Google Scholar]
- Flores, M.S.R.; Escuredo, O.; Seijo, M.C. Assessment of physicochemical and antioxidant characteristics of Quercus pyrenaica honeydew honeys. Food Chem. 2015, 166, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Ouchemoukh, S.; Ouchemoukh, N.A.; Romero, M.G.; Aboud, F.; Giuseppe, A.; Gutierrez, A.F.; Carretero, A.S. Characterisation of phenolic compounds in Algerian honeys by RP-HPLC coupled to electrospray time-of-flight mass spectrometry. LWT Food Sci. Technol. 2017, 85, 460–469. [Google Scholar] [CrossRef]
- Zerrouk, S.; Seijo, M.C.; Boughediri, L.; Escuredo, O.; Rodríguez-Flores, M.S. Palynological characterisation of Algerian honeys according to their geographical and botanical origin. Grana 2014, 53, 147–158. [Google Scholar] [CrossRef]
- Oddo, L.P.P.; Piana, L.; Bogdanov, S.; Bentabol, A.; Gotsiou, P.; Kerkvliet, J.; Martin, P.; Morlot, M.; Ortiz Valbuena, A.; Ruoff, K.; et al. Botanical species giving unifloral honey in Europe. Apidologie 2004, 35, S82–S93. [Google Scholar] [CrossRef]
- Bonvehi, J.S.; Manzanares, A.B.; Vilar, J.M.S. Quality evaluation of broom honey (Spartocytisus supranubius L.) produced in Tenerife (The Canary Islands). J. Sci. Food Agric. 2004, 84, 1097–1104. [Google Scholar] [CrossRef]
- Galloni, M.; Cristofolini, G. Floral rewards and pollination in Cytiseae (Fabaceae). Plant Syst. Evol. 2003, 238, 127–137. [Google Scholar] [CrossRef]
- Dimou, M.; Thrasyvoulou, A. Pollen analysis of honeybee rectum as a method to record the bee pollen flora of an area. Apidologie 2009, 40, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Berrekia, R.A.; Abdelguerfi, A.; Bounaga, N.; Guittonneau, G.G. Répartition des espèces spontanées du genre Hedysarum selon certains facteurs du milieu en Algérie. Fourrages 1991, 126, 187–207. [Google Scholar]
- Chefrour, E.; Draiaia, R.; Tahar, A.; Kaki, Y.A.; Bennadja, S.; Battesti, M.J. Physicochemical characteristics and pollen spectrum of some north-east Algerian honeys. Afr. J. Food Agric. Nutr. Dev. 2009, 9, 1276–1293. [Google Scholar] [CrossRef]
- Makhloufi, C.; Kerkvliet, J.; Schweitzer, P. Characterisation of some monofloral Algerian honeys by pollen analysis. Grana 2015, 54, 156–166. [Google Scholar] [CrossRef]
- Nair, S.; Meddah, B.; Aoues, A. Melissopalynological Characterization of North Algerian Honeys. Foods 2013, 2, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamel, T.; Boulemtafes, A. Plants foraged by bees in the Edough peninsula (Northeast Algeria). Livest. Res. Rural Dev. 2017, 29, 1–13. [Google Scholar]
- Hamel, T.; Bellili, M.; Hamza, A.M.; Boulemtafes, A. Nouvelle contribution à l’étude de la flore mellifère et caractérisation pollinique de miels de la Numidie (Nord-Est algérien). Livest. Res. Rural Dev. 2019, 31, 1–24. [Google Scholar]
- Escuredo, O.; Silva, L.R.; Valentão, P.; Seijo, M.C.; Andrade, P.B. Assessing Rubus honey value: Pollen and phenolic compounds content and antibacterial capacity. Food Chem. 2012, 130, 671–678. [Google Scholar] [CrossRef]
- Piana, M.L.; Oddo, L.P.; Bentabol, A.; Bruneau, E.; Bogdanov, S.; Declerck, C.G. Sensory analysis applied to honey: State of the art. Apidologie 2004, 35, S26–S37. [Google Scholar] [CrossRef] [Green Version]
- Araujo, D.; Cacho, P.R.P.; Serrano, S.; Palomares, R.D.; Soldevilla, H.G. Sensory Profile and Physico-Chemical Properties of Artisanal Honey from Zulia, Venezuela. Foods 2020, 9, 339. [Google Scholar] [CrossRef] [Green Version]
- Flores, M.S.R.; Falcão, S.I.; Escuredo, O.; Seijo, M.C.; Vilas-Boas, M. Description of the volatile fraction of Erica honey from the northwest of the Iberian Peninsula. Food Chem. 2021, 336, 127758. [Google Scholar] [CrossRef]
- Floris, I.; Satta, A.; Ruiu, L. Honeys of Sardinia (Italy). J. Apic. Res. 2007, 46, 198–209. [Google Scholar] [CrossRef]



| Sample | Area | Harvest Period | Altitude (a.m.s.l.) |
|---|---|---|---|
| M01 | Timsyet (Tizi N’berber) | Summer | 312 |
| M02 | Draa El-Gaid | Summer | 580 |
| M03 | Ighil Hassan | Summer | 320 |
| M04 | Lota village | Summer | 20 |
| M05 | Tahalaket (Tichy) | Summer | 167 |
| M06 | Ijouyaze Sahel | Summer | 205 |
| M07 | Adrar Oufarnou | Summer | 280 |
| M08 | Ait Aissa, Aokas | Summer | 185 |
| M09 | Djermana (Aokas) | Summer | 250 |
| M10 | Agwni Oukouche (Melbou) | Summer | 596 |
| M11 | Ouagaz Ichaabanen (Aokas) | Summer | 245 |
| M12 | Annar Assam | Summer | 335 |
| M13 | Annar Assam | Summer | 335 |
| M14 | Timsyet (Tizi N’berber) | Summer | 312 |
| M15 | Tahalaket | Spring | 167 |
| M16 | Tizi N’berber | Summer | 350 |
| M17 | Ouagaz Ichaabanen | Summer | 245 |
| M18 | Mechta Ledjbel (Ziama) | Summer | 590 |
| M19 | Draa El-Gaid | Summer | 580 |
| M20 | Tabelout | Summer | 305 |
| M21 | Tasabounet | Summer | 220 |
| M22 | Zentout-Tamrijet | Summer | 290 |
| M23 | Melbou | Summer | 03 |
| M24 | Ait Idir-Adekar | Summer | 500 |
| M25 | Tizi Ahmed | Summer | 330 |
| M26 | Agounane-Derguina | Summer | 300 |
| M27 | Djoa-Ajloh (Tichy) | Summer | 260 |
| M28 | AiT Anane-Derguina | Summer | 80 |
| M29 | Tababort | Summer | 600 |
| M30 | Boukhlifa | Summer | 280 |
| Sensory Perception | Descriptors |
|---|---|
| Estate | Liquid, Crystallized |
| Color | White, Straw, Gold, Orange, Brown |
| Smell | Vegetal, Floral, Animal, Chemical, Fruity, Degraded |
| Taste | Sweetness, Bitterness, Saltiness, Sourness |
| Aroma | Vegetal, Floral, Animal, Chemical, Fruity, Degraded |
| Tertiary attributes | Astringency, Spicy |
| Family | Pollen Type | Max. (%) | D (≥45%) | A (45–15%) | R (15–3%) | I (3–1%) | Present 1 |
|---|---|---|---|---|---|---|---|
| Amaryllidaceae | Allium t. | 5.7 | - | - | 1 | - | 11 |
| Anacardiaceae | Pistacia | 30.0 | - | 2 | 7 | 4 | 22 |
| Apiaceae | Daucus carota t. | 3.9 | - | - | 2 | 2 | 7 |
| Apiaceae | Foeniculum vulgare t. | 9.0 | - | - | 5 | 6 | 22 |
| Apiaceae | Pimpinella anisum t. | 6.0 | - | - | 3 | 4 | 15 |
| Asparagaceae | Asparagus acutifolius | 21.0 | - | 1 | - | - | 1 |
| Asteraceae | Aster t. | 39.7 | - | 1 | 3 | 5 | 15 |
| Asteraceae | Galactites t. | 6.9 | - | - | 3 | 3 | 21 |
| Boraginaceae | Echium | 4.4 | - | - | 2 | 6 | 17 |
| Capparaceae | Capparis spinosa | 12.7 | - | - | 1 | - | 4 |
| Cistaceae | Cistus t. | 11.7 | - | - | 1 | 6 | 19 |
| Cyperaceae | Carex | 5.0 | - | - | 1 | 2 | 6 |
| Ericaceace | Erica | 72.8 | 4 | 7 | 7 | 4 | 25 |
| Fabaceae | Astragalus | 22.1 | - | 1 | - | - | 1 |
| Fabaceae | Ceratonia siliqua | 7.3 | - | - | 1 | 1 | 2 |
| Fabaceae | Genista t | 73.9 | 3 | 15 | 8 | 1 | 29 |
| Fabaceae | Hedysarum | 63.2 | 1 | - | 7 | 6 | 20 |
| Fabaceae | Lotus t | 7.3 | - | - | 4 | 5 | 21 |
| Fabaceae | Spartium junceum t. | 16.8 | - | 1 | 5 | 1 | 8 |
| Fabaceae | Trifolium repens t. | 17.9 | - | 1 | 10 | 4 | 26 |
| Fagaceae | Castanea | 17.1 | - | 1 | 1 | - | 2 |
| Fagaceae | Quercus | 7.6 | - | - | 2 | 2 | 12 |
| Lamiaceae | Stachys t. | 9.7 | - | - | 6 | 7 | 21 |
| Lythraceae | Punica granatum | 10.5 | - | - | 1 | 3 | 5 |
| Myrtaceae | Eucalyptus | 41.9 | - | 2 | 4 | 4 | 16 |
| Myrtaceae | Myrtus communis | 25.9 | - | 3 | 12 | 4 | 24 |
| Oleaceae | Olea europaea | 6.1 | - | - | 1 | 1 | 4 |
| Rosaceae | Crataegus t. | 7.1 | - | - | 4 | 2 | 8 |
| Rosaceae | Prunus t. | 4.9 | - | - | 2 | 1 | 12 |
| Rosaceae | Rubus | 58.7 | 1 | 1 | 3 | 7 | 21 |
| Salicaceae | Salix | 11.6 | - | - | 4 | 1 | 11 |
| Tamaricaceae | Tamarix | 5.7 | - | - | 1 | 1 | 4 |
| Asteraceae | Rubus | Heather | Sulla | Genista | Honeydew | Polyfloral | |
|---|---|---|---|---|---|---|---|
| (n = 1) | (n = 1) | (n = 6) | (n = 2) | (n = 2) | (n = 1) | (n = 17) | |
| Main Pollen (%) | Aster t | Rubus | Erica | Hedysarum | Genista t | ||
| 39.8 | 58.7 | 54.2 ± 16.9 | 58.8 ± 6.2 | 72.0 ± 2.6 | |||
| N. Pollen Types | 31 | 19 | 19 ± 4 | 19 ± 6 | 20 ± 2 | 18 | 27 ± 6 |
| PK (pollen/g) | 13686 | 5292 | 7248 ± 4192 | 11110 ± 11338 | 4174 ± 2982 | 14465 | 5118 ± 5440 |
| Maurizio classes | III | II | II, III | III | II | III | I, II, III |
| Humidity (%) | 16.8 | 19.2 | 18.4 ± 0.6 | 18.1 ± 2.4 | 17.9 ± 0.7 | 19.6 | 17.9 ± 1.0 |
| EC (mS/cm) | 0.57 | 0.82 | 0.75 ± 0.2 | 0.54 ± 0.3 | 0.33 ± 0.0 | 1.35 | 0.55 ± 0.2 |
| pH | 3.7 | 3.5 | 4.0 ± 0.2 | 3.6 ± 0.2 | 3.9 ± 0.0 | 4.4 | 3.8 ± 0.1 |
| Free acidity | 33.5 | 35.5 | 34.7 ± 10.2 | 39.0 ± 2.8 | 15.5 ± 0.7 | 38.5 | 32.8 ± 8.2 |
| Color (mm Pfund) | 52 | 58 | 122 ± 7 | 53 ± 23 | 53 ± 22 | 135 | 66 ± 17 |
| Light Amber | Light Amber | Dark Amber | Light Amber | Light Amber | Dark Amber | Light Amber | |
| Diastase Index | 8.5 | 7.6 | 7.5 ± 2.2 | 14.8 ± 2.8 | 8.6 ± 2.4 | 16.9 | 12.5 ± 5.9 |
| HMF (mg/100 g) | 10.3 | 10.5 | 4.9 ± 0.8 | 7.0 ± 5.2 | 4.1 ± 0.5 | 5.4 | 11.5 ± 7.9 |
| Polyphenols (mg/100 g) | 49.6 | 53.9 | 109.8 ± 16.4 | 52.6 ± 7.8 | 52.6 ± 11.2 | 128.3 | 60.5 ± 15.8 |
| Flavonoids (mg/100 g) | 3.3 | 3.6 | 7.9 ± 1.3 | 3.8 ± 1.6 | 3.2 ± 1.3 | 8.7 | 4.1 ± 1.3 |
| Na (mg/100 g) | 9.4 | 11.9 | 12.0 ± 4.6 | 9.1 ± 5.1 | 6.9 ± 1.9 | 9.8 | 11.5 ± 5.9 |
| K (mg/100 g) | 122.4 | 170.5 | 193 ± 60.5 | 105.5 ± 71.4 | 66.9 ± 33.6 | 394.4 | 109.5 ± 55.7 |
| P (mg/100 g) | 27.6 | 23.9 | 25.7 ± 1.8 | 26.6 ± 4.8 | 20.0 ± 3.4 | 36.1 | 25.8 ± 4.1 |
| Ca (mg/100 g) | 6.2 | 4.0 | 12.9 ± 4.8 | 6.2 ± 3.2 | 2.9 ± 0.7 | 14.7 | 6.1 ± 1.6 |
| Mg (mg/100 g) | 2.1 | 2.9 | 10.7 ± 3.5 | 2.7 ± 1.7 | 2.9 ± 1.8 | 11.2 | 4.3 ± 2.6 |
| Fe (mg/100 g) | 0.2 | 0.1 | 0.3 ± 0.2 | 0.3 ± 0.1 | 0.15 ± 0.1 | 1.3 | 0.15 ± 0.1 |
| Mn (mg/100 g) | <0.2 | <0.2 | <0.35 | <0.2 | <0.2 | 0.4 | <0.2 |
| Cu (mg/100 g) | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| Zn (mg/100 g) | 0.1 | 0.1 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 | 0.1 ± 0.1 |
| Cd (mg/100 g) | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Pb (mg/100 g) | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghorab, A.; Rodríguez-Flores, M.S.; Nakib, R.; Escuredo, O.; Haderbache, L.; Bekdouche, F.; Seijo, M.C. Sensorial, Melissopalynological and Physico-Chemical Characteristics of Honey from Babors Kabylia’s Region (Algeria). Foods 2021, 10, 225. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10020225
Ghorab A, Rodríguez-Flores MS, Nakib R, Escuredo O, Haderbache L, Bekdouche F, Seijo MC. Sensorial, Melissopalynological and Physico-Chemical Characteristics of Honey from Babors Kabylia’s Region (Algeria). Foods. 2021; 10(2):225. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10020225
Chicago/Turabian StyleGhorab, Asma, María Shantal Rodríguez-Flores, Rifka Nakib, Olga Escuredo, Latifa Haderbache, Farid Bekdouche, and María Carmen Seijo. 2021. "Sensorial, Melissopalynological and Physico-Chemical Characteristics of Honey from Babors Kabylia’s Region (Algeria)" Foods 10, no. 2: 225. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10020225