A Detailed Characterisation of Appetite, Sensory Perceptional, and Eating-Behavioural Effects of COVID-19: Self-Reports from the Acute and Post-Acute Phase of Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Recruitment
2.2. Online Survey
2.3. Data Analysis
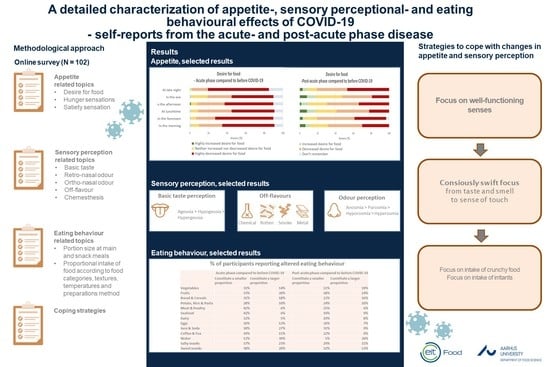
3. Results
3.1. Appetite
3.1.1. Desire for Food
3.1.2. Time of Day
3.1.3. Hunger and Satiety Sensations
3.2. Sensory Perception
3.2.1. Basic Taste Perception and Intake of Food with a Dominant Basic Taste
3.2.2. Orthonasal and Retronasal Odour Perception
3.2.3. Off-Flavour
3.2.4. Chemesthesis
3.3. Eating Behaviour
3.3.1. Portion Size of Main Meals and Snacks
3.3.2. Type of Diet
3.3.3. Texture, Temperature, and Preparation Method
3.4. Handling of Changes in Appetite, Sensory Perception, and Food Behaviour
4. Discussion
4.1. Altered Appetite
4.2. Sensory Perception
4.3. Eating Behaviour
4.4. Limitations
4.5. Application of Findings and Suggestions for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novelcoronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19#:~:text=symptoms (accessed on 14 January 2021).
- Grant, M.C.; Arbyn, M.; Mohammed, Z.; McGuinness, L.; Clarke, E.L.; Wade, R.G. The prevalence of symptoms in 24,410 individuals infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries. PLoS ONE 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter european study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Lechien, J.R.; Cabaraux, P.; Chiesa-Estomba, C.M.; Khalife, M.; Hans, S.; Calvo-Henriquez, C.; Martiny, D.; Journe, F.; Sowerby, L.; Saussez, S. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck 2020, 42, 1583–1590. [Google Scholar] [CrossRef]
- Spinato, G.; Fabbris, C.; Polesel, J.; Cazzador, D.; Borsetto, D.; Hopkins, C.; Boscolo-Rizzo, P. Alterations in smell or taste in mildly symptomatic outpatients with sars-cov-2 infection. JAMA 2020, 323, 2089–2090. [Google Scholar] [CrossRef] [Green Version]
- Menni, C.; Valdes, A.M.; Freidin, M.B.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Ganesh, S.; Varsavsky, T.; Cardoso, M.J.; Moustafa, J.S.E.-S.; et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020, 26, 1037–1040. [Google Scholar] [CrossRef]
- Beltran-Corbellini, A.; Chico-Garcia, J.L.; Martinez-Poles, J.; Rodriguez-Jorge, F.; Natera-Villalba, E.; Gomez-Corral, J.; Gomez-Lopez, A.; Monreal, E.; Parra-Dıaz, P.; Cortes-Cuevas, J.L.; et al. Acute-onset smell and taste disorders in the context of COVID-19: A pilot multicentre polymerase chain reaction based case–control study. Eur. J. Neurol. 2020, 9, 1738–1741. [Google Scholar] [CrossRef]
- Parma, V.; Ohla, K.; Veldhuizen, M.G.; Niv, M.Y.; Kelly, C.E.; Bakke, A.J.; Cooper, K.W.; Bouysset, C.; Pirastu, N.; Dibattista, M. More than smell–COVID-19 is associated with severe impairment of smell, taste and, chemesthesis. Chem. Senses 2020, 45, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Hopkins, C.; Salzano, G.; Petrocelli, M.; Melis, A.; Cucurullo, M.; Ferrari, M.; Gagliardini, L.; Pipolo, C.; Deiana, G.; et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck 2020, 42, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Deiana, G.; Fois, A.G.; Pirina, P.; Madeddu, G.; De Vito, A.; Babudieri, S.; Petrocelli, M.; Serra, A.; Bussu, F.; et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck 2020, 42, 1252–1258. [Google Scholar] [CrossRef]
- Eliezer, M.; Hautefort, C.; Hamel, A.-L.; Verillaud, B.; Herman, P.; Houdart, E.; Eloit, C. Sudden and complete olfactory loss function as a possible symptom of COVID-19. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 674–675. [Google Scholar] [CrossRef] [Green Version]
- Moein, S.T.; Hashemian, S.M.; Mansourafshar, B.; Khorram-Tousi, A.; Tabarsi, P.; Doty, R.L. Smell dysfunction: A biomarker for COVID-19, in ‘International forum of allergy & rhinology. Wiley Online Libr. 2020, 10, 944–950. [Google Scholar]
- Prajapati, D.P.; Shahrvini, B.; MacDonald, B.V.; Crawford, K.L.; Lechner, M.; DeConde, A.S.; Yan, C.H. Association of subjective olfactory dysfunction and 12-item odor identification testing in ambulatory COVID-19 patients, in ‘International forum of allergy & rhinology. Wiley Online Libr. 2020, 10, 1209–1217. [Google Scholar]
- Long-Term Health Effects of COVID-19. Available online: https://www.sst.dk/en/English/Corona-eng/COVID9-and-novel-coronavirus/Long-term-effects-of-COVID-19 (accessed on 14 January 2021).
- Andersen, B.V.; Hyldig, G. Consumers’ view on determinants to food satisfaction. A qualitative approach. Appetite 2015, 95, 9–16. [Google Scholar] [CrossRef]
- Andersen, B.V.; Hyldig, G. Food satisfaction: Integrating feelings before, during and after food intake. Food Qual. Prefer. 2015, 43, 126–134. [Google Scholar] [CrossRef]
- Harris, R. Life’s a smelling success: Using scent to empower your memory and learning. Int. J. Arimatherapy 2005, 15, 58–59. [Google Scholar] [CrossRef]
- Spinelli, S.; Monteleone, E.; Ares, G.; Jaeger, S.R. Sensory drivers of product-elicited emotions are moderated by liking: Insights from consumer segmentation. Food Qual. Prefer. 2019, 78, 1–12. [Google Scholar] [CrossRef]
- Spinelli, S.; Jaeger, S.R. What do we know about the sensory drivers of emotions in food and beverages? Curr. Opin. Food Sci. 2019, 27, 82–89. [Google Scholar] [CrossRef]
- Høier, A.T.Z.B.; Chaaban, N.; Andersen, B.V. Possibilities for maintaining appetite in recovering COVID-19 patients. Foods 2021, 10, 464. [Google Scholar] [CrossRef]
- Healthy AtHome: Healthy Diet. Available online: https://www.who.int/campaigns/connecting-the-world-tocombat-coronavirus/healthyathome/healthyathome---healthy-diet (accessed on 14 January 2021).
- Butowt, R.; von Bartheld, C.S. Anosmia in COVID-19: Underlying Mechanism and Assessment of an Olfactory Route to Brain Infection. Neuroscientist 2020. [Google Scholar] [CrossRef]
- Han, A.Y.; Mukdad, L.; Long, J.L.; Lopez, I.A. Anosmia in COVID-19: Mechanisms and Significance. Chem. Senses 2020, 45, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bai, W.; Hashikawa, T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020, 92, 552. [Google Scholar] [CrossRef]
- Murray, M.; Vickers, Z. Consumer views of hunger and fullness. A qualitative approach. Appetite 2009, 53, 174–182. [Google Scholar] [CrossRef]
- Eccles, R. Mechanisms and Symptoms of Common Cold and Flu; Eccles, R., Weber, O., Eds.; Common cold. Birkhäuser Verlag: Basel, Switzerland, 2009; pp. 23–45. [Google Scholar]
- Phan, L. Clinical Characteristics of COVID-19 Patients with Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020, 115, 766–773. [Google Scholar]
- McCrickerd, K.; Forde, C.G. Sensory influences on food intake control: Moving beyond palatability. Obes. Rev. 2016, 17, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Forde, C.G. From perception to ingestion; the role of sensory properties in energy selection, eating behaviour and food intake. Food Qual. Prefer. 2018, 66, 171–177. [Google Scholar] [CrossRef]
- Yeomans, M.R. Palatability and the Micro-structure of Feeding in Humans: The Appetizer Effect. Appetite 1996, 27, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Merkonidis, C.; Grosse, F.; Ninh, T.; Hummel, C.; Heahner, A.; Hummel, T. Characteristics of chemosensory disorders–results from a survey. Eur. Arch. Otorhinolaryngol. 2014, 272, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A. A learning model of binge eating: Cue reactivity and cue exposure. Behav. Res. Ther. 1998, 36, 257–272. [Google Scholar] [CrossRef]
- Nederkoorn, C.; Smulders, F.; Havermans, R.; Jansen, A. Exposure to binge food in bulimia nervosa: Finger pulse amplitude as a potential measure of urge to eat and predictor of food intake. Appetite 2004, 42, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Whitelock, V.; Robinson, E. Remembered meal satisfaction, satiety and later snack food intake: A laboratory study. Nutrients 2018, 10, 1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duerlund, M.; Andersen, B.V.; Byrne, D.V. Sensory specific desires affect and are affected by actual snack choice. J. Food Nutr. Health 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Neto, D.B.; Fornazieri, M.A.; Dib, C.; Francesco, R.C.D.; Doty, R.L.; Voegels, R.L.; Pinna, F.D.R. Chemosensory dysfunction in Covid-19: Prevalences, recovery rates, and clinical associations on large Brazilian sample. Otolaryngol. Head Neck Surg. 2020, 164, 512–518. [Google Scholar] [CrossRef]
- Niklassen, A.S.; Draf, J.; Huart, C.; Hintschich, C.; Bocksberger, S.; Trecca, E.M.C.; Klimek, L.; Bon, S.D.L.; Altundag, A.; Hummel, T. Covid-19: Recovery from chemosensory Dysfunction. A multicentre study on smell and taste. Laryngoscope 2021. [Google Scholar] [CrossRef]
- Huart, C.; Philpott, C.; Konstantinidis, I.; Altundag, A.; Whitcroft, K.L.; Trecca, E.M.C.; Cassano, M.; Rombaux, P.; Hummel, T. Comparison of Covid-19 and common cold chemosensory dysfunction. Rhinology 2020, 58, 623–625. [Google Scholar]
- Brugliera, L.; Spina, A.; Castellazzi, P.; Cimino, P.; Arcuri, P.; Negro, A.; Houdayer, E.; Alemanno, F.; Giordani, A.; Mortini, P.; et al. Nutritional management of COVID-19 patients in rehabilitation unit. Eur. J. Clin. Nutr. 2020, 74, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Thibault, R.; Seguin, P.; Tamion, F.; Pichard, C.; Singer, P. Nutrition of the COVID-19 in the intensive care uni (ICU): A practical guidance. Crit Care 2020, 24, 447. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, H.R.; Krieger, B. The contribution to sensory liking to overall liking: An analysis of six food categories. Food Qual. Prefer. 1995, 6, 85–90. [Google Scholar] [CrossRef]
- Andersen, B.V.; Brockhoff, P.B.; Hyldig, G. The importance of liking of appearance, -odour, -taste and -texture in the evaluation of overall liking. A comparison with the evaluation of sensory satisfaction. Food Qual. Prefer. 2019, 71, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Moskowitz, H.; Krieger, B. What sensory characteristics drive product quality? An assessment of individual differences. J. Sens. Stud. 1992, 8, 271–282. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Mahmut, M.K.; Hortsmann, A.; Hummel, T. The Aetiology of Olfactory Dysfunction and Its Relationship to Diet Quality. Brain Sci. 2020, 10, 769. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, J.C.; Mattes, R.D. Nutrition and taste and smell dysfunction. World J. Otorhinolaryngol. Head Neck Surg. 2018, 4, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, K.; Hummel, C.; Teszmer, K.; Krone, F.; Ishimaru, T.; Seo, H.-S.; Hummel, T. The influence of olfactory loss on dietary behaviors. Laryngoscope 2007, 118, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Kong, I.; Kim, S.; Prk, B.; Kim, J.; Choi, H. Olfactory dysfunction is associated with the intake of macronutrients in Korean adults. PLoS ONE 2016, 11, e0164495. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Han, P.; Burghardt, S.; Knaapila, A.; Schriever, V.; Hummel, T. Influence of olfactory dysfunction on the perception of food’. Eur. Arch. Otorhinolaryngol. 2019, 276, 2811–2817. [Google Scholar] [CrossRef] [PubMed]











| Characteristics | |
|---|---|
| Total number (n) | 102 |
| Males/females | 14/88 |
| Age (years) | 41 ± 12.9 (19–69) * |
| BMI (kg/m2) | 25.7 ± 5 (17.8–42.9) * |
| Educational level (min–max) ** | 4.7 ± 1.3 (2–6) * |
| Inhabitants/household (number of persons) | 3 ± 1.4 (1–8) * |
| Response Variable | During the Acute Phase | During the Post-Acute Phase |
|---|---|---|
| Desire for food | ‘During the acute phase, how large was your desire for food compared to before COVID-19?’ | ‘While you are in the post-acute phase, how large has your desire for food been recently compared to before COVID-19’ |
| Hunger | ‘Indicate how COVID-19 affected following hunger sensations, x, during the acute phase (compared to before COVID-19)’ x = ‘desire to eat’,’ stomach churning’, ‘empty stomach feeling’, ‘stomach pain’, ‘lack of energy’, ‘thoughts circulating around food’ and ‘shaking sensation’ | ‘Now that you are in the post-acute phase, how will you assess following hunger sensations, x, compared to before COVID-19?’ x = ‘desire to eat’, ‘stomach churning’, ‘empty stomach feeling’, ‘stomach pain’, ‘lack of energy’, ‘thoughts circulating around food’ and ‘shaking sensation’ |
| Satiety | ‘Indicate how COVID-19 affected following satiety sensations, x, during the acute phase (compared to before COVID-19)’ x = ‘general satiety’, ‘post-meal satisfaction’, ‘feeling bloated’, ‘heavy stomach feeling’, ‘nausea’, ‘energetic’ and ‘difficulty breathing’ | ‘Now that you are in the post-acute phase, how will you assess following satiety sensations, x, compared to before COVID-19?’ x = ‘general satiety’, ‘post-meal satisfaction’, ‘feeling bloated’, ‘heavy stomach feeling’, ‘nausea’, ‘energetic’ and ‘difficulty breathing’ |
| Taste perception | ‘During the acute phase, how did you experience the intensity of the x taste?’ x = ‘sweet’, ‘salty’, ‘sour’ and ‘bitter’ | ‘How are you experiencing the intensity of the x taste lately?’ x = ‘sweet’, ‘salty’, ‘sour’ and ‘bitter’ |
| Retronasal odour perception | ‘Did COVID-19 change your ability to perceive flavours?’ ‘How did the changes ability of perceiving flavour affect your desire for eating?’ | |
| Off-flavour perception | ‘Did COVID-19 cause any off-flavours in your mouth?’ ‘How did these off-flavours affect your desire for eating?’ | |
| Orthonasal odour perception | ‘Did COVID-19 change your ability to perceive odours?’ ‘How did the changes in the ability of perceiving odours affect your desire for eating?’ | |
| Chemesthetic perception * | ‘Did COVID-19 cause any changes in the sense of touch during food intake?’ ‘How did these feelings affect your desire for eating?’ | |
| Quantitative food intake | The participants were asked to indicate the portion size of their daily meals (x) during the acute phase compared to before COVID-19. x = ‘breakfast’, ‘pre-lunch snack’, ‘lunch’, ‘afternoon snack’, ‘dinner’, ‘late night snack’ | The participants were asked to indicate the portion size of their current daily meals (x) compared to before COVID-19. x = ‘breakfast’, ‘pre-lunch snack’, ‘lunch’, ‘afternoon snack’, ‘dinner’, ‘late night snack’ |
| Qualitative food intake | The participants were asked to indicate to what extent a certain food and type of food, x, were part of their diet during the acute phase compared to before COVID-19. x = ‘vegetables’, ‘fruits’, ‘bread and cereal’, ‘pasta, rice and potato’, ‘meat, meat products and poultry’, ‘seafood’, ‘dairy products’, ‘eggs’, ‘juice and soda’, ‘coffee and tea’, ‘water’, ‘salty snacks’ and ‘sweet snacks’ | The participants were asked to indicate to what extent a certain food and type of food, x, are part of their diet currently compared to before COVID-19. x = ‘vegetables’, ‘fruits’, ‘bread and cereal’, ‘pasta, rice and potato’, ‘meat, meat products and poultry’, ‘seafood’, ‘dairy products’, ‘eggs’, ‘juice and soda’, ‘coffee and tea’, ‘water’, ‘salty snacks’ and ‘sweet snacks’ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaaban, N.; Høier, A.T.Z.B.; Andersen, B.V. A Detailed Characterisation of Appetite, Sensory Perceptional, and Eating-Behavioural Effects of COVID-19: Self-Reports from the Acute and Post-Acute Phase of Disease. Foods 2021, 10, 892. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10040892
Chaaban N, Høier ATZB, Andersen BV. A Detailed Characterisation of Appetite, Sensory Perceptional, and Eating-Behavioural Effects of COVID-19: Self-Reports from the Acute and Post-Acute Phase of Disease. Foods. 2021; 10(4):892. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10040892
Chicago/Turabian StyleChaaban, Nora, Alexander Teymour Zadeh Baboli Høier, and Barbara Vad Andersen. 2021. "A Detailed Characterisation of Appetite, Sensory Perceptional, and Eating-Behavioural Effects of COVID-19: Self-Reports from the Acute and Post-Acute Phase of Disease" Foods 10, no. 4: 892. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10040892