Chemosensory Device Assisted-Estimation of the Quality of Edible Oils with Repetitive Frying
Abstract
:1. Introduction
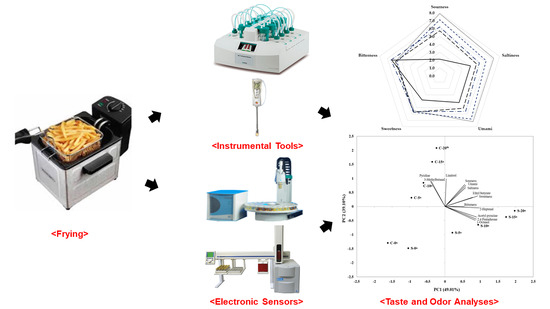
2. Materials and Methods
2.1. Material
2.2. Chemical Measures
2.3. Sensory Measures
2.4. Statistical Analyses
3. Results
3.1. Chemical Properties
3.2. Sensory Properties
3.3. Multivariate Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Saleh, A.S.M.; Chen, J.; Shen, Q. Chemical alterations taken place during deep-fat-frying based on certain reaction products: A review. Chem. Phys. Lipids 2012, 165, 662–681. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Ghazani, S.M.; Marangoni, A.G. Quality and safety of frying oils used in restaurants. Food Res. Int. 2014, 64, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Gertz, C. Fundamentals of the frying process. Eur. J. Lipid Sci. Technol. 2014, 116, 669–674. [Google Scholar] [CrossRef]
- Gunstone, F. Vegetable Oils in Food Technology: Composition, Properties and Uses, 2nd ed.; John Willey & Sons: New York, NY, USA, 2011; pp. 59–136. [Google Scholar]
- Moreira, R.G.; Castell-Perez, M.E.; Barrufet, M.A. Frying Oil Characteristics: Deep-Fat Frying Fundamentals and Applications; Aspen Publishers Inc.: Gaithersburg, ML, USA, 1999; pp. 33–74. [Google Scholar]
- Zhang, Q.; Qin, W.; Li, M.; Qun, S.; Saleh, A.S.M. Application of chromatographic techniques in the detection and identification of constituents formed during food frying: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 601–633. [Google Scholar] [CrossRef] [Green Version]
- Przybylski, R.; Gruczynska, E.; Aladedunye, F. Performance of Regular and Modified Canola and Soybean Oils in Rotational Frying. J. Am. Oil Chem. Soc. 2013, 90, 1271–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Budge, S.M. Techniques for the analysis of minor lipid oxidation products derived from triacylglycerols: Epoxides, alcohols, and ketons. Compr. Rev. Food Sci. Food Saf. 2017, 16, 735–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Park, J.M.; Kim, H.J.; Koh, J.H.; Kim, J.M. Physicochemical changes in edible oils (soybean, canola, palm, and lard) and fried foods (pork cutlet and potato) depending on fry number. Korean J. Food Sci. Technol. 2017, 49, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Kim, D.S.; Cho, J.J.; Hong, S.J.; Bu, C.G.; Shin, E.C. Oxidative stability, physicochemical, and sensory characteristics of vegetable oils at their induction periods. J. Korean Soc. Food Sci. Nutr. 2019, 48, 649–660. [Google Scholar] [CrossRef]
- Choe, E.; Min, B.D. Chemistry of deep-fat frying oils. J. Food Sci. 2007, 72, 77–86. [Google Scholar] [CrossRef]
- Xu, X.Q.; Tran, V.H.; Palmer, M. Chemical and physical analyses and sensory evaluation of six deep-frying oils. J. Am. Oil Chem. Soc. 1999, 76, 1091–1099. [Google Scholar]
- Matthäus, B. Utilization of high-oleic rapeseed oil for deep-fat frying of French fries compared to other commonly used edible oils. Eur. J. Lipid Sci. Technol. 2006, 108, 200–211. [Google Scholar] [CrossRef]
- Santos, C.; García, L.; Cunha, S.; Casal, S. Fried potatoes: Impact of prolonged frying in monounsaturated oils. Food Chem. 2017, 243. [Google Scholar] [CrossRef] [Green Version]
- Majchrzak, T.; Wojnowski, W.; Dymerski, T.; Gebicki, J.; Namiesnik, J. Electronic nose in classification and quality control of edible oils: A review. Food Chem. 2018, 246, 192–201. [Google Scholar] [CrossRef]
- Men, H.; Chen, D.; Zhang, X.; Liu, J.; Ning, K. Data fusion of electronic nose and electronic tongue for detection of mixed edible-oil. J. Sens. 2014, 2014, 840685. [Google Scholar] [CrossRef] [Green Version]
- Ross, C.F. Considerations of the use of the electronic tongue in sensory science. Curr. Opin. Food Sci. 2021, 40, 87–93. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.; Huang, J.; Gao, P.; Jin, Q.; Wang, X. Rapid Measuring Flavor Quality Changes of Frying Rapeseed Oils using a Flash Gas Chromatography Electronic Nose. Eur. J. Lipid Sci. Technol. 2019, 121, 1800260. [Google Scholar] [CrossRef]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Portable Electronic Nose Based on Electrochemical Sensors for Food Quality Assessment. Sensors 2017, 24, 2715. [Google Scholar] [CrossRef] [Green Version]
- Innawong, B.; Mallikarjunan, P.; Marcy, J.E. The determination of frying oil quality using a chemosensory system. LWT 2004, 37, 35–41. [Google Scholar] [CrossRef]
- Costa, A.M.S.; Sobral, M.M.C.; Delgadillo, I.; Rudnitskaya, I. Electronic tongue as a rapid tool for the assessment of coffee flavour and chemical composition. Sensors 2014, 2126–2129. [Google Scholar] [CrossRef]
- Rodríguez-Méndez María, L.; De Saja José, A.; González-Antón, R.; García-Hernández, C.; Medina-Plaza, C.; García-Cabezón, C.; Martín-Pedrosa, F. Electronic Noses and Tongues in Wine Industry. Front. Bioeng. Biotechnol. 2016, 4, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, Y.; Meng, Q.; Li, N.; Ren, L. Evaluation of Beef by Electronic Tongue System TS-5000Z: Flavor Assessment, Recognition and Chemical Compositions According to Its Correlation with Flavor. PLoS ONE 2015, 10, e0137807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOCS. AOCS Official and Tentative Methods, 10th ed.; American Oil Chemists’ Society: Chicago, IL, USA, 1990; AOCS Official Method Cd 30-63. [Google Scholar]
- AOCS. AOCS Official and Tentative Methods, 10th ed.; American Oil Chemists’ Society: Chicago, IL, USA, 1990; AOCS Official Method Cd 8-53. [Google Scholar]
- Durán Merás, I.; Periañez Llorente, C.; Pilo Ramajo, J.; Martín Tornero, E.; Espinosa-Mansilla, A. Optimization of the thiobarbituric acid-malonaldehyde reaction in non-aqueous medium. Direct analysis of malonaldehyde in oil samples by HPLC with fluorimetric detection. Microchem. J. 2020, 159, 105318. [Google Scholar]
- Noh, B.S. Analysis of volatile compounds using electronic nose and its application in food industry. Korean J. Food Sci. Technol. 2005, 35, 1048–1064. [Google Scholar]
- Deisingh, A.K.; Stone, D.C.; Thompson, M. Applications of electronic noses and tongues in food analysis. Int. J. Food Sci. Technol. 2004, 39, 587–604. [Google Scholar] [CrossRef]
- Kim, J.S.; Jung, H.Y.; Park, E.Y.; Noh, B.S. Flavor Analysis of Commercial Korean Distilled Spirits using an Electronic Nose and Electronic Tongue. Korean J. Food Sci. Technol. 2016, 48, 117–121. [Google Scholar] [CrossRef]
- Jo, Y.H.; Gu, S.Y.; Chung, N.H.; Gao, Y.; Kim, H.J.; Jeong, M.H.; Jeong, Y.J.; Kwon, J.H. Comparative analysis of sensory profiles of commercial cider vinegars from Korea, China, Japan, and US by SPME/GC-MS, E-nose, and E-tongue. Korean J. Food Sci. Technol. 2016, 48, 430–436. [Google Scholar] [CrossRef] [Green Version]
- Legin, A.; Rudnitskaya, A.; Vlasov, Y.; Di Natale, C.; Mazzone, E.; D’Amico, A. Application of electronic tongue for quantitative analysis of mineral water and wine. Electroanal 1999, 11, 814–820. [Google Scholar] [CrossRef]
- Anwar, F.; Hussain, A.; Iqbal, S.; Bhanger, M. Enhancement of the oxidative stability of some vegetable oils by blending with Moringa oleifera oil. Food Chem. 2007, 103, 1181–1191. [Google Scholar] [CrossRef]
- Katragadda, H.; Fullana, A.; Sidhu, S.; Carbonell-Barrachina, A. Emissions of volatile aldehydes from heated cooking oils. Food Chem. 2010, 120, 59–65. [Google Scholar] [CrossRef]
- de Alzaa, F.; Guillaume, C.; Ravetti, L. Evaluation of Chemical and Physical Changes in Different Commercial Oils during Heating. Acta Sci. 2018, 2, 2–11. [Google Scholar]
- Kim, H.S.; Kim, D.S.; Lee, J.; Hong, S.J.; Cho, J.J.; Woo, S.M.; Shin, E.C. Characterization of edible oil containing wasabi during frying process. J. Korean Soc. Food Sci. Nutr. 2018, 47, 1191–1199. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, M.J.; Kim, Y.J.; Lee, J.H. Monitoring changes in acid value, total polar material, and antioxidant capacity of oils used for frying chicken. Food Chem. 2017, 220, 306–312. [Google Scholar] [CrossRef]
- Bansal, G.; Zhou, W.; Barlow, P.; Lo, H.; Neo, F. Performance of palm olein in repeated deep frying and controlled heating processes. Food Chem. 2010, 121, 338–347. [Google Scholar] [CrossRef]
- Rouseff, R.; Gmitter, F.; Grosser, J. Citrus breeding and flavor. In Understanding Natural Flavors; Piggott, J.R., Paterson, A., Eds.; Springer: Boston, MA, USA, 1994; pp. 113–127. [Google Scholar]
- Burdock, G. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 1090–1216. [Google Scholar]
- Xu, Q. Electronic nose for characterization of flavour patters of deep frying oils. Food Aust. 2006, 58, 89–91. [Google Scholar]
- Reineccius, G. Flavor technology. In Flavor Chemistry and Technology, 2nd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 103–137. [Google Scholar]
- Jo, Y.; Benoist, D.M.; Ameerally, A.A.; Drake, M.A. Sensory and chemical properties of Gouda cheese. Int. J. Dairy Sci. 2018, 101, 1967–1989. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Huang, Z.; Qi, X.; Li, Y.; Zhang, G.; Lei, A. Copper-catalyzed aerobic oxidative coupling: From ketone and diamine to pyrazine. Sci. Adv. 2015, 1, e1500656. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, R.; Mishra, H.N. Multivariate analysis for kinetic modeling of oxidative stability and shelf life estimation of sunflower oil blended with sage (Salvia officinalis) extract under rancimat conditions. Food Bioprocess Tech. 2015, 8, 801–810. [Google Scholar] [CrossRef]
- Llorent-Martinex, E.J.; Ortega-Barrales, P.; Fernandez-de Cordova, M.L.; Dominguez-Vidal, A.; Ruiz-Medina, A. Investigation by ICP-MS of trace element levels in vegetable edible oils produced in Spain. Food Chem. 2011, 127, 1257–1262. [Google Scholar] [CrossRef]
- Xu, L.; Yu, X.; Liu, L.; Zhang, R. A novel method for qualitative analysis of edible oil oxidation using an electronic nose. Food Chem. 2016, 202, 229–235. [Google Scholar] [CrossRef]



| Oils | Induction Time (hr) | Acid Value (mg KOH/g) | p-Anisidine Value | Malondialdehyde (meq/kg) | Total Polar Compounds (%) |
|---|---|---|---|---|---|
| Soybean-0th | 3.02 a ± 0.04 1) | 0.09 d ± 0.01 | 16.2 e ± 1.63 | 0.18 e ± 0.02 | 9.67 c ± 0.04 |
| Soybean-5th | 1.86 b ± 0.06 | 0.24 c ± 0.04 | 23.6 d ± 0.18 | 0.54 d ± 0.02 | 11.5 b ± 0.41 |
| Soybean-10th | 1.12 c ± 0.04 | 0.36 b ± 0.02 | 29.0 c ± 0.34 | 0.87 c ± 0.02 | 11.8 b ± 0.23 |
| Soybean-15th | 0.82 d ± 0.05 | 0.47 ab ± 0.06 | 34.4 b ± 1.98 | 1.24 b ± 0.04 | 12.0 b ± 0.01 |
| Soybean-20th | 0.81 d ± 0.04 | 0.52 a ± 0.01 | 40.3 a ± 1.81 | 1.65 a ± 0.00 | 13.0 a ± 0.02 |
| Canola-0th | 4.93 a ± 0.10 | 0.06 e ± 0.02 | 17.5 c ± 2.30 | 0.10 e ± 0.02 | 4.67 d ± 0.12 |
| Canola-5th | 2.42 b ± 0.15 | 0.15 d ± 0.01 | 34.1 b ± 0.87 | 1.02 d ± 0.03 | 6.17 c ± 0.23 |
| Canola-10th | 1.76 c ± 0.21 | 0.28 c ± 0.02 | 35.1 b ± 0.69 | 1.39 c ± 0.03 | 7.33 b ± 0.25 |
| Canola-15th | 1.52 c ± 0.05 | 0.36 b ± 0.01 | 41.0 a ± 1.14 | 1.81 b ± 0.03 | 8.00 ab ± 0.10 |
| Canola-20th | 1.39 c ± 0.19 | 0.44 a ± 0.01 | 44.5 a ± 1.22 | 2.82 a ± 0.04 | 8.67 a ± 0.24 |
| Oils | 2,4-Pentadinone | Ethyl Butyrate | 2-Heptenal | Acetyl Pyrazine | 1-Octanol | Linalool | 3-Methylbutanal | Pyridine |
|---|---|---|---|---|---|---|---|---|
| Soybean-0th | 38.23 d ± 3.25 | 20.66 e ± 1.88 | 36.44 c ± 1.93 | 20.32 d ± 1.56 | 15.33 c ± 1.25 | 17.66 d ± 1.21 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Soybean-5th | 78.22 c ± 6.28 | 130.2 d ± 9.42 | 100.3 b ± 8.56 | 43.41 c ± 3.32 | 20.56 ± 2.02 | 36.13 c ± 3.01 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Soybean-10th | 95.53 b ± 7.12 | 200.8 c ± 14.32 | 115.9 ab ± 7.56 | 50.04 b ± 4.04 | 23.23 b ± 2.06 | 40.52 b ± 3.34 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Soybean-15th | 115.5 a ± 10.99 | 230.4 b ± 13.97 | 121.1 a ± 11.24 | 53.07 b ± 3.88 | 30.51 a ± 3.11 | 43.98 b ± 3.21 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Soybean-20th | 118.4 a ± 8.35 | 260.5 a ± 18.90 | 135.3 a ± 13.90 | 60.64 a ± 3.22 | 32.38 a ± 3.69 | 50.63 a ± 4.28 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Canola-0th | 0.00 ± 0.00 | 18.51 d ± 1.12 | 3.23 c ± 0.42 | 5.58 e ± 0.23 | 0.00 ± 0.00 | 16.78 d ± 1.21 | 5.03 d ± 0.34 | 2.76 d ± 0.12 |
| Canola-5th | 0.00 ± 0.00 | 85.36 c ± 6.32 | 36.34 b ± 2.54 | 12.26 d ± 1.05 | 0.00 ± 0.00 | 50.02 c ± 4.23 | 25.07 c ± 1.98 | 21.67 c ± 2.86 |
| Canola-10th | 0.00 ± 0.00 | 120.4 b ± 9.47 | 47.92 ab ± 3.18 | 15.44 c ± 1.23 | 0.00 ± 0.00 | 80.46 b ± 7.73 | 28.35 b ± 3.23 | 25.82 b ± 2.13 |
| Canola-15th | 0.00 ± 0.00 | 165.8 a ± 12.42 | 55.78 a ± 4.89 | 18.08 b ± 1.47 | 0.00 ± 0.00 | 102.6 a ± 9.21 | 32.72 b ± 2.78 | 29.64 a ± 2.06 |
| Canola-20th | 0.00 ± 0.00 | 173.5 a ± 17.02 | 61.43 a ± 6.09 | 22.21 a ± 1.99 | 0.00 ± 0.00 | 110.1 a ± 8.77 | 37.87 a ± 2.22 | 28.74 ± 2.32 |
| Sourness | Saltiness | Umami | Sweetness | Bitterness | 2,3-Pentanedione | Ethyl Butyrate | 2-Heptenal | Acetyl pyrazine | 1-Octanol | Linalool | 3-Methylbutanal | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saltiness | 0.87 * | |||||||||||
| Umami | 0.87 * | 0.85 * | ||||||||||
| Sweetness | 0.65 | 0.52 | 0.73 | |||||||||
| Bitterness | 0.24 | 0.08 | 0.42 | 0.57 | ||||||||
| 2,3-Pentanedione | 0.16 | 0.25 | 0.18 | 0.53 | 0.41 | |||||||
| Ethyl butyrate | 0.82 * | 0.79 | 0.82 * | 0.81 * | 0.53 | 0.65 | ||||||
| 2-Heptenal | 0.55 | 0.59 | 0.49 | 0.68 | 0.40 | 0.90 * | 0.88 * | |||||
| Acetyl pyrazine | 0.24 | 0.26 | 0.20 | 0.56 | 0.41 | 0.97 * | 0.69 | 0.92 * | ||||
| 1-Octanol | 0.09 | 0.21 | 0.14 | 0.50 | 0.37 | 0.99 * | 0.59 | 0.87 * | 0.95 * | |||
| Linalool | 0.80 * | 0.73 | 0.75 | 0.31 | −0.04 | −0.40 | 0.40 | 0.01 | −0.32 | −0.44 | ||
| 3-Methylbutanal | 0.49 | 0.34 | 0.42 | 0.01 | −0.20 | −0.77 | −0.05 | −0.44 | −0.71 | −0.80 * | 0.87 * | |
| Pyridine | 0.51 | 0.33 | 0.43 | 0.04 | −0.20 | −0.75 | −0.03 | −0.42 | −0.66 | −0.78 | 0.87 * | 0.99 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Boo, C.; Hong, S.-j.; Shin, E.-C. Chemosensory Device Assisted-Estimation of the Quality of Edible Oils with Repetitive Frying. Foods 2021, 10, 972. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10050972
Lee J, Boo C, Hong S-j, Shin E-C. Chemosensory Device Assisted-Estimation of the Quality of Edible Oils with Repetitive Frying. Foods. 2021; 10(5):972. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10050972
Chicago/Turabian StyleLee, Jookyeong, Changguk Boo, Seong-jun Hong, and Eui-Cheol Shin. 2021. "Chemosensory Device Assisted-Estimation of the Quality of Edible Oils with Repetitive Frying" Foods 10, no. 5: 972. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10050972