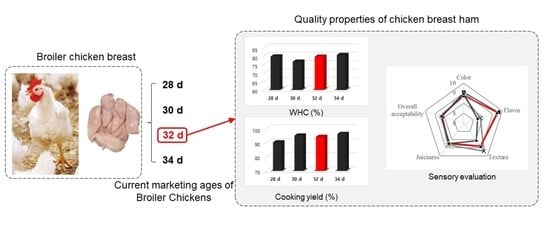
Effects of Marketing Ages on the Physicochemical Properties and Sensory Aspects of Cured Broiler Chicken Breast Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cured Chicken Breast Manufacture
2.2. Proximate Composition
2.3. pH
2.4. Water-Holding Capacity (WHC)
2.5. Curing Yield
2.6. Cooking Yield
2.7. Color
2.8. Shear-Force
2.9. Electronic Nose
2.10. Electronic Tongue
2.11. Sensory Evaluation
2.12. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition
3.2. pH and Water Holding Capacity (WHC)
3.3. Curing and Cooking Yield
3.4. Color
3.5. Shear-Force
3.6. Aromatic and Taste Profiles
3.7. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kim, G.W.; Kim, J.H.; Kim, H.Y.; Kim, B.K.; Park, H.B.; Choe, J.; Kim, J.H. Analysis of marketing performances according to raising environment in broilers. Korean J. Poult. Sci. 2019, 46, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Nasr, J.; Kheiri, F. Increasing amino acids density improves broiler live weight. Int. J. Poult. Sci. 2011, 10, 523–526. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Byeon, D.S.; Kim, G.W.; Kim, H.Y. Carcass and retail meat cuts quality properties of broiler chicken meat based on the slaughter age. J. Anim. Sci. Technol. 2021, 63, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Goliomytis, M.; Panopoulou, E.; Rogdakis, E. Growth curves for body weight and major component parts, feed consumption, and mortality of male broiler chickens raised to maturity. Poult. Sci. 2003, 82, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Frischknecht, C.O.; Jull, M.A. Amount of breast meat and live and dressed grades in relation to body measurements in 12-week old purebred and crossbred chickens. Poult. Sci. 1946, 25, 330–345. [Google Scholar] [CrossRef]
- Migineishvili, A. Criteria of selection for higher broiler chicken breast yield. In Quality of Poultry Products: Poultry Meat, 1st ed.; Veerkamp, C.H., Uijttenboogaarr, T.G., Eds.; Spelderholt centre for poultry research and information services: Beekbergen, The Netherlands, 1991; pp. 235–241. [Google Scholar]
- Beski, S.S.M.; Swick, R. Specialized protein products in broiler chicken nutrition: A review. Anim. Nutr. 2015, 1, 47–53. [Google Scholar] [CrossRef]
- Wattanachant, S.; Benjakul, S.; Ledward, D.A. Composition, color, and texture of Thai indigenous and broiler chicken muscles. Poult. Sci. 2004, 83, 123–128. [Google Scholar] [CrossRef]
- Font-i-Furnols, M.; Guerrero, L. Consumer preference, behavior and perception about meat and meat products: An overview. Meat Sci. 2014, 98, 361–371. [Google Scholar] [CrossRef]
- Ali, M.; Lee, S.Y.; Park, J.Y.; Jung, S.; Jo, C.; Nam, K.C. Comparison of functional compounds and micronutrients of chicken breast meat by breeds. Food Sci. Anim. Resour. 2019, 4, 632–642. [Google Scholar] [CrossRef]
- Nagai, H.; Harada, M.; Nakagawa, M.; Tanaka, T.; Gunadi, B.; Justinus Setiabudi, M.L.; Uktolseja, J.L.; Miyata, Y. Effects of chicken extract on the recovery from fatigue caused by mental workload. Appl. Hum. Sci. 1996, 15, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, H.; Savage, E.M.; Kays, S.E.; Himmelsbach, D.S. A survey of the quality of six retail brands of boneless, skinless chicken breast fillets obtained from retail supermarkets in the Athens, Georgia area. J. Food Qual. 2007, 30, 1068–1082. [Google Scholar] [CrossRef]
- Andrée, S.; Jira, W.; Schwind, K.H.; Wagner, H.; Schwägele, F. Chemical safety of meat and meat products. Meat Sci. 2010, 86, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Froning, G.W.; Sackett, B. Effect of salt and phosphates during tumbling of turkey breast muscle on meat characteristics. Poult. Sci. 1985, 64, 1328–1333. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990; pp. 777–788. [Google Scholar]
- Grau, R.; Hamm, R. Eine einfache methode zur bestimmung der wasserbinding in muskel. Naturwissenschaften 1953, 40, 29. [Google Scholar] [CrossRef]
- Park, S.Y.; Seol, K.H.; Kim, H.Y. Effect of dry-aged beef crust levels on quality properties of brown sauce. Food Sci. Anim. Resour. 2020, 40, 699–709. [Google Scholar] [CrossRef]
- Taylor, R.G. Connective tissue structure, function and influence on meat quality. In Encyclopedia of Meat Sciences, 1st ed.; Jensen, W.K., Devine, C., Dikeman, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 306–314. [Google Scholar]
- Prockop, D.J. Collagens. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, W.J., Daniel Lane, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 545–549. [Google Scholar]
- Abougabal, M.S.; Taboosha, M.F. Productive performance, carcass characteristics and meat quality of broiler chickens at different marketing ages. Egypt. Poult. Sci. 2020, 40, 275–289. [Google Scholar] [CrossRef] [Green Version]
- Berri, C.; Le Binam Duval, E.; Baeza, E.; Chartrin, P.; Picgirard, L.; Jehl, N.; Quentin, M.; Picard, M.; Duclos, M.J. Further processing characteristics of breast and leg meat from fast-, medium- and slow-growing commercial chickens. Anim. Res. 2005, 54, 123–134. [Google Scholar] [CrossRef]
- Petracci, M.; Cavani, C. Muscle growth and poultry meat quality issues. Nutrients 2012, 4, 1–12. [Google Scholar] [CrossRef]
- Smith, D.P.; Fletcher, D.L. Chicken breast muscle fiber type and diameter as influenced by age and intramuscular location. Poult. Sci. 1988, 67, 908–913. [Google Scholar] [CrossRef]
- Połtowicz, K.; Doktor, J. Effect of slaughter age on performance and meat quality of slow-growing broiler chickens. Ann. Anim. Sci. 2012, 12, 621–631. [Google Scholar] [CrossRef] [Green Version]
- Berri, C.; Le Bihan-Duval, E.; Sante´-Lhoutellier, V.; Bae´za, E.; Gigaud, V.; Je´go, Y.; Duclos, M.J. Consequence of muscle hypertrophy on characteristics of Pectoralis major muscle and breast meat quality of broiler chickens. J. Anim. Sci. 2007, 85, 2005–2011. [Google Scholar] [CrossRef] [Green Version]
- Karunanayaka, D.S.; Jayasena, D.D.; Jo, C. Prevalence of pale, soft, and exudative (PSE) condition in chicken meat used for commercial meat processing and its effect on roasted chicken breast. J. Anim. Sci. Technol. 2016, 58, 27. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, G.F. On the relationship of ultrastructural and cytochemical features to color in mammailan skeletal muscle. Cell Tissue Res. 1969, 95, 462–482. [Google Scholar]
- Brooke, M.H.; Kaiser, K.K. Muscle fiber types: How many and what kind? Arch. Neurol. 1970, 23, 369–379. [Google Scholar] [CrossRef]
- Ashmore, C.R.; Doerr, L. Comparative aspects of muscle fiber types in different species. Exp. Neurol. 1971, 31, 408–418. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.; Kim, H.Y. Physicochemical characteristics of breast and thigh meats from old broiler breeder hen and old laying hen and their effects on quality properties of pressed ham. Poult. Sci. 2020, 99, 2230–2235. [Google Scholar] [CrossRef] [PubMed]
- Krause, R.J.; Ockerman, H.W.; Krol, B.; Moerman, P.C.; Plimpton, R.F., Jr. Influence of tumbling, tumbling time, trim and sodium tripolyphosphate on quality and yield of cured hams. J. Food Sci. 1978, 43, 853–855. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choi, J.H.; Choi, Y.S.; Kim, H.Y.; Ahn, K.I.; Kim, H.W.; Kim, T.H.; Song, D.H.; Kim, C.J. Effects of low-temperature tumbling on the quality characteristics of restructured chicken breast ham. Korean J. Food Sci. Ani. Resour. 2012, 32, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef]
- Renerre, M. Factors involved in the discoloration of beef meat. Int. J. Food Sci. Technol. 1990, 25, 613–630. [Google Scholar] [CrossRef]
- Keeton, J.T.; Eddy, S. Chemical and physical characteristics of meat. In Encyclopedia of Meat Sciences, 1st ed.; Jensen, W.K., Devine, C., Dikeman, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 210–265. [Google Scholar]
- Wasserman, A.E. Thermally produced flavor components in the aroma of meat and poultry. J. Agric. Food Chem. 1972, 20, 737–741. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lolenzo, J.M. A comprehensive review of lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Marion, J.E.; Woodroof, J.G. The fatty acid composition of breast, thigh, and skin tissue of chicken broilers as influenced by dietary fats. Poult. Sci. 1963, 42, 1202–1207. [Google Scholar] [CrossRef]
- Okumura, T.; Saito, K.; Sowa, T.; Sakuma, H.; Ohhashi, F.; Tameoka, N.; Hirayama, M.; Nakayama, S.; Sato, S.; Gogami, T.; et al. Changes in beef sensory traits as somatic-cell-cloned Japanese black steers increased in age from 20 to 30 months. Meat Sci. 2012, 90, 159–163. [Google Scholar] [CrossRef]
- Liu, X.D.; Jayasena, D.D.; Jung, Y.; Jung, S.; Kang, B.S.; Heo, K.N.; Lee, J.H.; Jo, C. Differential proteome analysis of breast and thigh muscles between Korean native chickens and commercial broilers. Asian Austral. J. Anim. Sci. 2012, 25, 895–902. [Google Scholar] [CrossRef]
- Sebranek, J.G.; Bacus, J.N. Cured meat products without direct addition of nitrate of nitrite: What are the issues? Meat Sci. 2007, 77, 136–147. [Google Scholar] [CrossRef]
- Rikimaru, K.; Takahashi, H. Evaluation of the meat from Hinai-jidori chickens and broilers: Analysis of general biochemical components, free amino acids, inosine 5′-monophosphate, and fatty acids. Appl. Poult. Res. 2010, 19, 327–333. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jung, S.; Kim, H.J.; Yong, H.I.; Nam, K.C.; Jo, C. Taste-active compound levels in Korean native chicken meat: The effects of bird age and the cooking process. Poult. Sci. 2015, 94, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, D.D.; Ahn, D.U.; Nam, K.C.; Jo, C. Flavour chemistry of chicken meat: A review. Asian Austral. J. Anim. Sci. 2013, 26, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, H.S.; Richards, J.F. Flavor of poultry meat—A review. Can. Inst. Food Technol. J. 1982, 15, 7–18. [Google Scholar] [CrossRef]





| Traits (%) | Marketing Ages (day) | |||
|---|---|---|---|---|
| 28 | 30 | 32 | 34 | |
| Water | 70.62 ± 0.10 a | 68.79 ± 0.77 a,b | 68.85 ± 0.09 a,b | 68.13 ± 1.26 b |
| Protein | 21.09 ± 2.53 b | 24.45 ± 0.17 a,b | 24.42 ± 0.64 a,b | 24.91 ± 0.34 a |
| Fat | 0.95 ± 0.12 | 1.00 ± 0.08 | 1.01 ± 0.07 | 1.11 ± 0.14 |
| Ash | 2.50 ± 0.04 | 2.50 ± 0.06 | 2.57 ± 0.14 | 2.48 ± 0.08 |
| Traits | Marketing Ages (d) | ||||
|---|---|---|---|---|---|
| 28 | 30 | 32 | 34 | ||
| pH | Uncooked | 5.85 ± 0.01 c | 5.86 ± 0.01 c | 5.93 ± 0.01 b | 5.95 ± 0.01 a |
| Cooked | 6.14 ± 0.01 d | 6.18 ± 0.01 c | 6.20 ± 0.01 b | 6.26 ± 0.01 a | |
| WHC (%) | 90.17 ± 0.13 b | 94.98 ± 0.45 a | 94.02 ± 1.21 a | 96.09 ± 1.48 a | |
| Traits | Marketing Ages (day) | |||
|---|---|---|---|---|
| 28 | 30 | 32 | 34 | |
| CIE L* (lightness) | 70.68 ± 0.55 a | 69.90 ± 0.81 a | 67.22 ± 0.64 b | 63.70 ± 0.68 c |
| CIE a* (redness) | 3.83 ± 0.12 b | 3.82 ± 0.20 b | 3.77 ± 0.15 b | 4.65 ± 0.26 a |
| CIE b* (yellowness) | 18.25 ± 0.44 c | 19.19 ± 0.14 b | 19.27 ± 0.90 b | 20.40 ± 0.75 a |
| Hue angle (H°) | 74.14 ± 0.58 a | 78.74 ± 0.53 a | 78.90 ± 0.91 a | 77.16 ± 0.61 b |
| Chroma (C*) | 18.65 ± 0.42 c | 19.57 ± 0.17 b | 19.64 ± 0.86 b | 20.92 ± 0.76 a |
| Items | Marketing Ages (day) | |||
|---|---|---|---|---|
| 28 | 30 | 32 | 34 | |
| AHS_sourness (1) | 3.76 | 8.41 | 6.61 | 5.23 |
| PKS | 6.28 | 5.99 | 5.89 | 5.83 |
| CTS_saltiness | 5.38 | 5.87 | 5.99 | 6.76 |
| NMS_umami | 5.39 | 5.91 | 5.91 | 6.79 |
| CPS | 5.81 | 5.77 | 5.88 | 6.54 |
| ANS | 8.04 | 3.99 | 5.51 | 6.46 |
| SCS | 8.12 | 3.24 | 5.53 | 7.10 |
| Traits | Marketing Ages (day) | |||
|---|---|---|---|---|
| 28 | 30 | 32 | 34 | |
| Color | 8.86 ± 0.42 | 9.04 ± 0.56 | 8.88 ± 0.39 | 8.96 ± 0.58 |
| Flavor | 8.36 ± 0.49 b | 9.30 ± 0.50 a | 9.24 ± 0.56 a | 8.22 ± 0.75 b |
| Texture | 9.20 ± 0.65 a | 8.92 ± 0.79 a,b | 8.90 ± 0.54 a,b | 8.56 ± 0.63 b |
| Juiciness | 8.82 ± 0.96 | 8.76 ± 0.58 | 8.70 ± 0.72 | 8.74 ± 0.61 |
| Taste | 8.80 ± 0.94 | 8.73 ± 0.60 | 8.68 ± 0.73 | 8.78 ± 0.64 |
| Overall acceptability | 8.66 ± 0.84 | 8.62 ± 0.68 | 8.70 ± 1.00 | 8.64 ± 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-Y.; Kim, H.-Y. Effects of Marketing Ages on the Physicochemical Properties and Sensory Aspects of Cured Broiler Chicken Breast Meat. Foods 2021, 10, 2152. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10092152
Park S-Y, Kim H-Y. Effects of Marketing Ages on the Physicochemical Properties and Sensory Aspects of Cured Broiler Chicken Breast Meat. Foods. 2021; 10(9):2152. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10092152
Chicago/Turabian StylePark, Sin-Young, and Hack-Youn Kim. 2021. "Effects of Marketing Ages on the Physicochemical Properties and Sensory Aspects of Cured Broiler Chicken Breast Meat" Foods 10, no. 9: 2152. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10092152