Phenolic Profile by HPLC-PDA-MS of Greek Chamomile Populations and Commercial Varieties and Their Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation of Phenolic Extracts
2.2. Chemicals
2.3. Standards
2.4. Determination of Total Phenolic and Total Flavonoid Contents
2.5. Determination of Antioxidant Activity by DPPH Assay
2.6. Determination of Antioxidant Activity by ABTS Assay
2.7. HPLC-PDA-MS Analysis Instrumentation
2.8. Qualitative and Quantitative Determination of Flavonoids and Caffeoyl Quinic Acids in the Herbal Drug
2.9. Statistical Analysis
3. Results
3.1. Identification of Phenolic Acids and Conjugates
3.2. Identification of Flavone and Flavonol Glycosides
3.3. Identification of Polyamine Conjugates
3.4. Total Phenolics, Total Flavonoid Content and Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Srivastava, J.K.; Gupta, S. Extraction, Characterization, Stability and Biological Activity of Flavonoids Isolated from Chamomile Flowers. Mol. Cell. Pharmacol. 2009, 1, 138. [Google Scholar] [CrossRef]
- Forster, H.B.; Niklas, H.; Lutz, S. Antispasmodic Effects of Some Medicinal Plants. Planta Medica 1980, 40, 309–319. [Google Scholar] [CrossRef] [PubMed]
- EMA. European Medicines Agency, Committee on Herbal Medicinal Products (HMPC). 2015. Available online: https://www.ema.europa.eu/documents/herbal-monograph/final-european-union-herbal-monograph-matricaria-recutita-l-flos_en.pdf (accessed on 8 September 2021).
- Raal, A.; Orav, A.; Püssa, T.; Valner, C.; Malmiste, B.; Arak, E. Content of essential oil, terpenoids and polyphenols in commercial chamomile (Chamomilla recutita L. Rauschert) teas from different countries. Food Chem. 2012, 131, 632–638. [Google Scholar] [CrossRef]
- Villa-Rodriguez, J.A.; Kerimi, A.; Abranko, L.; Tumova, S.; Ford, L.; Blackburn, R.S.; Rayner, C.; Williamson, G. Acute metabolic actions of the major polyphenols in chamomile: An in vitro mechanistic study on their potential to attenuate postprandial hyperglycaemia. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arihara, K. Functional Foods. In Encyclopedia of Meat Sciences, 2nd ed.; Dikeman, M., Devine, C., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 32–36. [Google Scholar]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria recutita L.): An overview. Pharmacogn. Rev 2011, 5, 82–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, V.; Mittal, P.; Bansal, P.; Khokra, S.L.; Kaushik, D. Pharmacological Potential of Matricaria recutita-A Review. Int. J. Pharm. Sci. Drug Res. 2010, 2, 12–16. [Google Scholar]
- Mao, J.J.; Xie, S.X.; Keefe, J.R.; Soeller, I.; Li, Q.S.; Amsterdam, J.D. Long-term Chamomile (Matricaria recutita L.) treatment for generalized anxiety disorder: A randomized clinical trial. Phytomedicine 2016, 23, 1735–1742. [Google Scholar] [CrossRef] [Green Version]
- Caleja, C.; Barros, L.; Antonio, A.L.; Ciric, A.; Barreira, J.C.M.; Sokovic, M.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Isabel Ferreira, I.C.F.R. Development of a functional dairy food: Exploring bioactive and preservation effects of chamomile (Matricaria recutita L.). J. Funct. Foods 2015, 16, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the Approximation of the Laws of the Member States Relating to Food Supplements. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32002L0046 (accessed on 8 September 2021).
- Ćwieląg-Drabek, M.; Piekut, A.; Szymala, I.; Oleksiuk, K.; Razzaghi, M.; Osmala, W.; Jabłońska, K.; Dziubanek, G. Health risks from consumption of medicinal plant dietary supplements. Food Sci. Nutr. 2020, 8, 3535–3544. [Google Scholar] [CrossRef] [PubMed]
- Coppens, P. The Use of Botanicals in Food Supplements and Medicinal Products: The Co-existence of two Legal Frameworks. Eur. Food Feed. Law Rev. 2008, 3, 93–100. [Google Scholar]
- Cassileth, B.R.; Heitzer, M.; Wesa, K. The public health impact of herbs and nutritional supplements. Pharm. Biol. 2009, 47, 761–767. [Google Scholar] [CrossRef] [Green Version]
- Chamomillae romanae flos-Roman Chamomile Flowers. In ESCOP Monographs, The Scientific Foundation for Herbal Medicinal Products; European Scientific Cooperative on Phytotherapy (ESCOP) Notaries House: Exeter, UK, 2019.
- EMA. EMA/HMPC/55837/2011. Committee on Herbal Medicinal Products (HMPC) Assessment Report on Matricaria recutita L., Flos and Matricaria recutita L., Aetheroleum. 2015. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-matricaria-recutita-l-flos-matricaria-recutita-l-aetheroleum_en.pdf (accessed on 8 September 2021).
- Pereira, S.V.; Reis, R.A.S.P.; Garbuio, D.C.; de Freitas, L.A.P. Dynamic maceration of Matricaria chamomilla inflorescences: Optimal conditions for flavonoids and antioxidant activity. Rev Bras Farm. 2018, 28, 111–117. [Google Scholar] [CrossRef]
- Fajemiroye, J.O.; Ferreira, N.L.; de Oliveira, L.P.; Elusiyan, C.A.; Pedrino, G.R.; da Cunha, L.C.; da Conceição, E.C. Matricaria recutita and its Isolate-Apigenin: Economic Value, Ethnopharmacology and Chemico-Biological Profiles in Retrospect. Res. Rev. J. Pharm. Phytochem. 2016, 4, 17–31. [Google Scholar]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. BioMed Res. Int. 2019, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel, F.G.; Cavalheiro, A.H.; Spinola, N.F.; Ribeiro, D.L.; Barcelos, G.R.M.; Antunes, L.M.G.; Hori, J.I.; Marquele-Oliveira, F.; Rocha, B.; Berretta, A.A. Validation of a RP-HPLC-DAD Method for Chamomile (Matricaria recutita) Preparations and Assessment of the Marker, Apigenin-7-glucoside, Safety and Anti-Inflammatory Effect. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Matricaria flower. Matricariae flos. In European Pharmacopoeia, 7th ed.; EDQM: Strasbourg, France, 2011; p. 1178.
- Svehliková, V.; Bennett, R.N.; Mellon, F.A.; Needs, P.W.; Piacente, S.; Kroon, P.A.; Bao, Y. Isolation, identification and stability of acylated derivatives of apigenin 7-O-glucoside from chamomile (Chamomilla recutita [L.] Rauschert). Phytochemistry 2004, 65, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Taviani, P.; Rosellini, D.; Veronesi, F. Variation for Agronomic and Essential Oil Traits Among Wild Populations of Chamomilla recutita (L.) Rauschert from Central Italy. J. Herbs Spices Med. Plants 2002, 9, 353–358. [Google Scholar] [CrossRef]
- Gosztola, B.; Németh, E.; Sárosi, S.; Szabó, K.; Kozák, A. Comparative evaluation of chamomile (Matricaria recutita L.) populations from different origin. Int. J. Hortic. Sci. 2006, 12, 91–95. [Google Scholar] [CrossRef]
- Tadrent, W.; Kabouche, A.; Touzani, R.; Kabouche, Z. Chemotypes investigation of essential oils of Chamomile herbs: A short review. J. Mater. Env. Sci. 2016, 7, 1229–1235. [Google Scholar]
- Tsivelika, N.; Sarrou, E.; Gusheva, K.; Pankou, C.; Koutsos, T.; Chatzopoulou, P.; Mavromatis, A. Phenotypic variation of wild Chamomile (Matricaria chamomilla L.) populations and their evaluation for medicinally important essential oil. Biochem. Syst. Ecol. 2018, 80, 21–28. [Google Scholar] [CrossRef]
- Avula, B.; Wang, Y.H.; Wang, M.; Avonto, C.; Zhao, J.; Smillie, T.J.; Rua, D.; Khan, I.A. Quantitative determination of phenolic compounds by UHPLC-UV–MS and use of partial least-square discriminant analysis to differentiate chemo-types of Chamomile / Chrysanthemum flower heads. J. Pharm. Biomed. Anal. 2014, 88, 278–288. [Google Scholar] [CrossRef]
- Vascular Plants Checklist of Greece. Available online: http://portal.cybertaxonomy.org/flora-greece/content (accessed on 5 September 2021).
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagents. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Irakli, M.; Mygdalia, A.; Chatzopoulou, P.; Katsantonis, D. Impact of combination of sourdough fermentation and hop extract addition on baking properties, antioxidant capacity and phenolics bioaccessibility of rice bran-enhanced bread. Food Chem. 2019, 285, 231–239. [Google Scholar] [CrossRef]
- Bao, J.; Cai, Y.; Sun, M.; Wang, G.; Corke, H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. J. Agric. Food Chem. 2005, 53, 2327–2332. [Google Scholar] [CrossRef]
- Yen, G.-C.; Chen, H.-Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ignatiadou, M.E.; Zoumpou, S.; Kabouche, Z.; Kostaki, M.; Rallis, M.; Karioti, A. Chemical analyzes of Matricaria pubescens and Matricaria recutita polar extracts and study of their anti-inflammatory properties. In Proceedings of the ISE 2021 Virtual Congress, Online. 18–20 April 2021. [Google Scholar]
- Justesen, U. Collision-induced fragmentation of deprotonated methoxylated flavonoids, obtained by electrospray ionization mass spectrometry. J. Mass Spectrom. 2001, 36, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Bolognesi, L.; Vincieri, F.F.; Bilia, A.R. Analysis of the constituents of aqueous preparations of Stachys recta by HPLC–DAD and HPLC–ESI-MS. J. Pharm. Biomed. Anal. 2010, 53, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the Six Isomers of Dicaffeoylquinic Acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Ultraviolet Spectra of Flavones and Flavonols. In The Systematic Identification of Flavonoids; Mabry, T.J., Markham, K.R., Thomas, M.B., Eds.; Springer: Berlin/Heidelberg, Germany, 1970; pp. 41–164. [Google Scholar]
- Lin, L.-Z.; Harnly, J.M. LC-PDA-ESI/MS Identification of the Phenolic Components of Three Compositae Spices: Chamomile, Tarragon, and Mexican Arnica. Nat. Prod. Commun. 2012, 7, 749–752. [Google Scholar] [CrossRef] [Green Version]
- Nováková, L.; Vildová, A.; Mateus, J.P.; Gonçalves, T.; Solich, P. Development and application of UHPLC–MS/MS method for the determination of phenolic compounds in Chamomile flowers and Chamomile tea extracts. Talanta 2010, 82, 1271–1280. [Google Scholar] [CrossRef]
- Repčák, M.; Pastirova, A.; Imrich, J.; Svehlikova, V.; Martonfi, P. The variability of (Z)-and (E)-2-b-D-glucopyranosyloxy-4-methoxycinnamic acids and apigenin glucosides in diploid and tetraploid Chamomilla recutita. Plant Breed 2001, 120, 188–190. [Google Scholar] [CrossRef]
- Mulinacci, N.; Romani, A.; Pinelli, P.; Vinceri, F.F.; Prucher, D. Characterization of Matricaria recutita L. flower extracts by HPLC-MS and HPLC-DAD analysis. Chromatographia 2000, 51, 301–307. [Google Scholar] [CrossRef]
- Franke, R.; Schilcher, H. Relevance and use of chamomile (Matricaria recutita L.). Acta Hort. 2007, 749, 29–43. [Google Scholar] [CrossRef]
- Jabeen, A.; Mesaik, M.A.; Simjee, S.U.; Lubna Bano, S.; Faizi, S. Anti-TNF-α and anti-arthritic effect of patuletin: A rare flavonoid from Tagetes patula. Int. Immunopharmacol. 2016, 36, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Minoshima, Y.; Yamamoto, J.; Adachi, I.; Watson, A.A.; Nash, R.J. Protective Effects of Dietary Chamomile Tea on Diabetic Complications. J. Agric. Food Chem. 2008, 56, 8206–8211. [Google Scholar] [CrossRef]
- Alvarado-Sansininea, J.J.; Sánchez-Sánchez, L.; López-Muñoz, H.; Escobar, M.L.; Flores-Guzmán, F.; Tavera-Hernández, R.; Jiménez-Estrada, M. Quercetagetin and Patuletin: Antiproliferative, Necrotic and Apoptotic Activity in Tumor Cell Lines. Molecules 2018, 23, 2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.B.; Song, K.; Kim, Y.S. Tetra-cis/trans-Coumaroyl Polyamines as NK1 Receptor Antagonists from Matricaria recutita. Planta Med. Int. Open 2017, 4, e43–e51. [Google Scholar]
- Redaelli, C.; Formentini, L.; Santaniello, E. Apigenin 7-glucoside and its 2″-and 6″-acetates from ligulate flowers of Matricaria chamomilla. Phytochemistry 1980, 19, 985–986. [Google Scholar] [CrossRef]
- Švehlíková, V.; Repčák, M. Variation of Apigenin Quantity in Diploid and Tetraploid Chamomilla recutita (L.) Rauschert. Plant Biol. 2000, 2, 403–407. [Google Scholar] [CrossRef]
- Faehnrich, B.; Nemaz, P.; Franz, C. Variability of certain chemotypes in three accessions of German chamomile (Matricaria recutita L.). Genet. Res Crop. Ev. 2014, 61, 1237–1244. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef] [Green Version]
- Rusaczonek, A.; Swiderski, F.; Waszkiewicz-Robak, B. Antioxidant properties of tea and herbal infusions–A short report. P. J. Food Nutr. Sci. 2010, 60, 33–35. [Google Scholar]
- Viapiana, A.; Struck-Lewicka, W.; Konieczynski, P.; Wesolowski, M.; Kaliszan, R. An Approach Based on HPLC-Fingerprint and Chemometrics to Quality Consistency Evaluation of Matricaria chamomilla L. Commercial Samples. Front. Plant Sci. 2016, 7, 1561. [Google Scholar] [CrossRef] [Green Version]
- Alibabaei, Z.; Rabiei, Z.; Rahnama, S.; Mokhtari, S.; Rafieian-kopaei, M. Matricaria chamomilla extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Biomed. Aging Pathol. 2014, 4, 355–360. [Google Scholar] [CrossRef]
- Mieriņa, I.; Jakaite, L.; Kristone, S.; Adere, L.; Jure, M. Extracts of Peppermint, Chamomile and Lavender as Antioxidants. Key Eng. Mater. 2018, 762, 31–35. [Google Scholar] [CrossRef]




| Rt (min) | UV (nm) | m/z (-) Negative Mode | m/z (+) Positive Mode | Identification | Mode of Identification | |
|---|---|---|---|---|---|---|
| 1 | 4.17 | 296, 326 | 191.1 [quinic acid-H]−, 352.9 [M-H]− | 163.0 [caffeic-H2O+H]+, 354.8 [M+H]+ | chlorogenic acid | UV/MS, std |
| 2 | 4.37 | 279, 301 | 134.1, 149.1, 193.1, 355.1 [M-H]−, 711.1 [2M-H]− | 177.2, 195.1, 378.8 [M+Na]+ | cis-2-hydroxy-4-methoxycinnamic-oxo-2-O-β-D-glucopyranoside | UV/MS, std, NMR |
| 3 | 7.88 | 259, 275sh, 354 | 317.0 [A-H]−, 478.9 [M-H]− | 318.9 [A+H]+, 481.0 [M+Na]- | quercetagenin-3-O-glucoside | UV/MS, std, NMR |
| 4 | 7.95 | 295, 318 | 134.1, 148.9, 193.0, 354.9 [M-H]−, 711.0 [2M-H]− | 379.0 [M+Na]+ | trans-2-hydroxy-4-methoxycinnamic-oxo-2-O-β-D-glucopyranoside | UV/MS, std, NMR |
| 5 | 11.62 | 255, 270sh, 370 | 300.9 [A-H]−, 463.1 [M-H]− | 303.0 [A+H]+, 464.7 [M+H]+ | quercetin-7-O-glucoside | UV/MS, std, NMR |
| 6 | 13.07 | 258, 274sh, 369 | 330.9 [A-H]−, 492.8 [M-H]− | 333.0 [A+H]+, 495.1 [M+H]+ | patuletin-7-O-glucoside | UV/MS, ref |
| 7 | 13.34 | 259, 275sh, 356 | 331.0 [A-H]−, 493.0 [M-H]− | 332.9 [A+H]+, 495.1 [M+H]+ | patuletin-3-O-glucoside | UV/MS, std, NMR |
| 8 | 14.48 | 255, 267sh, 347 | 284.9 [A-H]−, 446.9 [M-H]− | 287.1 [A+H]+, 449.1 [M+H]+ | luteolin-7-O-glucoside | UV/MS, std |
| 9 | 16.37 | 296, 325 | 173.1, 178.9, 191.1, 514.8 [M-H]− | 516.8 [M+H]+ | dicaffeoylquinic acid derivative | UV/MS |
| 10 | 17.85 | 254, 270sh, 370 | 314.9 [A-H]−, 477.1 [M-H]− | 316.9 [A+H]+, 479.0 [M+H]+ | isorhamnetin-7-O-glucoside | UV/MS, NMR |
| 11 | 18.23 | 254, 269sh, 352 | 313.8 [A-2H]−, 315.0 [A-H]−, 476.9 [M-H]− | 316.9 [A+H]+ | isorhamnetin-3-O-glucoside | UV/MS, std |
| 12 | 18.70 | 298, 327 | 179.1 [caffeic acid-H]−, 191.1 [quinic acid-H]−, 352.9 [M-caffeoyl-H]−, 514.9 [Μ-H]− | 539.0 [M+H]+ | 3,5-O-dicaffeoylquinic acid | UV/MS, std |
| 13 | 19.53 | 267, 336 | 268 [A-2H]−, 269.1 [A-H]−, 431.0 [M-H]− | 271.1 [A+H]+, 433.1 [M+H]+ | apigenin-7-O-glucoside | NMR/UV/MS, std |
| 14 | 20.53 | 252, 266sh, 347 | 298.7 [A-H]−, 445.9 [Μ-CH3-H]−, 460.9 [Μ-H]− | 301.0 [A+H]+, 463.0 [M+H]+ | chrysoeriol-7-O-glucoside | UV/MS, ref |
| 15 | 21.03 | 298, 327 | 172.9, 179.0 [caffeic acid-H]−, 191 [quinic acid-H]−, 353.0 [Μ-caffeoyl-H]−, 515.1 [Μ-H]− | 162.3 [caffeic acid-H2O +H]+ | 4,5-O-dicaffeoylquinic acid | UV/MS, std |
| 16 | 22.64 | 255, 267sh, 354 | 314.9 [A-H]− | 316.9 [A+H]+, 565.0 [M+H]+, 586.9 [M+Na]+ | isorhamnetin-3-O-malonylhexoside tentatively | UV/MS |
| 17 | 23.17 | 300, 319 | 160.8, 193.1, 517.3 [M-H]− | 163.0, 540.9 [M+Na]+ | acylated derivative of cis-2-hydroxy-4-methoxycinnamic-oxo-2-O-β-D-glucopyranoside | UV/MS |
| 18 | 25.66 | 254, 268sh, 367 | 315.1 [A-H]− | 317.1 [A+H]+, 565.2 [M+H]+ | isorhamnetin-7-O-malonylhexoside tentatively | UV/MS |
| 19 | 27.40 | 266, 330 | 269.1 [A-H]− | 271.1 [A+H]+, 519.1 [M+H]+ | apigenin-7-O-malonylhexoside | UV/MS |
| 20 | 28.21 | 267, 336 | 269.0 [A-H]−, 472.9 [M-CO2-H]− | 271.1 [A+H]+, 519.1 [M+H]+ | apigenin-7-O-(6′′-malonyl)-glucoside | UV/MS, ref |
| 21 | 29.74 | 267, 336 | 269.2 [A-H]−, 473.0 [M-H]− | 271.1 [A+H]+, 474.6 [M+H]+ | apigenin-7-acetylhexoside | UV/MS, ref |
| 22 | 30.62 | 298, 324 | 160.9, 193.1, 517.0 [M-H]− | - | acylated derivative of trans-2-hydroxy-4-methoxycinnamic-oxo-2-O-β-D-glucopyranoside | UV/MS |
| 23 | 36.28 | 267, 336 | 269.0 [A-H]−, 473.0 [M-H]− | 271.1 [A+H]+, 475.4 [M+H]+ | apigenin-7-acetylhexoside isomer | UV/MS, ref |
| 24 | 37.86 | 267, 336 | 269.0 [A-H]−, 515.0 [M-CO2-H]− | 271.1 [A+H]+, 561.1 [M+H]+ | apigenin-7-O-acetylmalonyl-hexoside | UV/MS, ref |
| 25 | 38.99 | 267, 334 | 268.9 [A-H]−, 515.2 [M-CO2-H]− | 271.1 [A+H]+, 560.9 [M+H]+ | apigenin-7-O-acetylmalonyl-hexoside | UV/MS, ref |
| 26 | 49.92 | 275 | 545.0, 665.1, 785.3 [M-H]− | 147.1 [coumaroyl+H]+, 641.3, 787.3 [M+H]+ | N1(Z)-N5(Z)-N10(Z)-N14(Z)-tetra-p-coumaroyl spermine/thermospermine (cis-isomers) | UV/MS, ref |
| 27 | 51.13 | 290 | 545.5, 665.1, 785.3 [M-H]− | 147.2 [coumaroyl+H]+, 641.2, 787.4 [M+H]+ | N1(Z)-N5(Z)-N9(Z)-N14(Z)- tetra-p-coumaroyl spermine/thermospermine (cis and trans-isomers) | UV/MS, ref |
| 28 | 52.01 | 292, 308 | 544.9, 665.1, 785.3 [M-H]− | 146.9 [coumaroyl+H]+, 641.3, 787.4 [M+H]+ | N1(E)-N5(E)-N10(E)-N14(E)- tetra-p-coumaroyl spermine (trans-isomer) | UV/MS, ref |
| 29 | 54.25 | 297, 308 | 545.1, 665.1, 785.3 [M-H]− | 147.1 [coumaroyl+H]+, 495.2 [M-2 x coumaroyl+H]+, 641.3 [M-coumaroyl+H]+, 787.5 [M+H]+, 809.3 [M+Na]+ | N1(E)-N5(E)-N9(E)-N14(E)- tetra-p-coumaroyl thermospermine (trans-isomer) | UV/MS, ref |
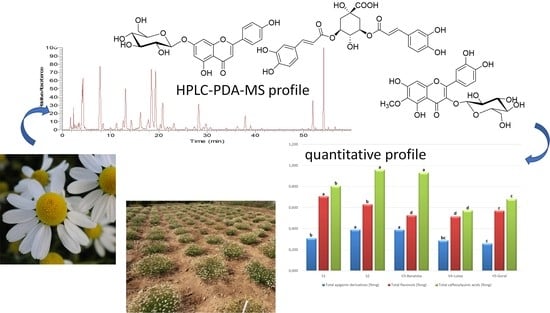
| S1 | S2 | V3-Banatska | V4-Lutea | V5-Goral | |
|---|---|---|---|---|---|
| Apigenin-7-O-glucoside | 0.147 ± 0.018 a | 0.17 ± 0.02 a | 0.17 ± 0.02 a | 0.10 ± 0.02 b | 0.10 ± 0.01 b |
| Apigenin-7-O-acetylglucoside | 0.108 ± 0.007 b | 0.15 ± 0.01 a | 0.15 ± 0.01 a | 0.08 ± 0.01 c | 0.07 ± 0.01 d |
| Apigenin-7-O-malonylacetylglucoside | 0.052 ± 0.002 c | 0.07 ± 0.01 b | 0.08 ± 0.01 b | 0.10 ± 0.01 a | 0.09 ± 0.01 a |
| Total apigenin derivatives | 0.31 ± 0.03 b | 0.39 ± 0.02 a | 0.40 ± 0.03 a | 0.28 ± 0.01 bc | 0.26 ± 0.02 c |
| Patuletin-3-O-glucoside | 0.49 ± 0.02 a | 0.45 ± 0.01 b | 0.22 ± 0.025 e | 0.25 ± 0.01 d | 0.30 ± 0.01 c |
| Patuletin-7-O-glucoside | 0.10 ± 0.01 d | 0.10 ± 0.01 d | 0.23± 0.01 a | 0.20 ± 0.01 c | 0.21 ± 0.01 b |
| Quercetagenin-3-O-glucoside | 0.11 ± 0.01 a | 0.09 ± 0.01 b | 0.08 ± 0.01 c | 0.06 ± 0.01 d | 0.06 ± 0.01 d |
| Total flavonols | 0.71 ± 0.02 a | 0.64 ± 0.01 b | 0.53 ± 0.02 d | 0.51 ± 0.01 d | 0.57 ± 0.03 c |
| 3,5-dicaffeoylquinic acid | 0.58 ± 0.04 c | 0.70 ± 0.02 a | 0.63 ± 0.03 b | 0.29 ± 0.01 e | 0.36 ± 0.01 d |
| 4,5-dicaffeoylquinic acid | 0.23 ± 0.02 d | 0.26 ± 0.01 c | 0.30 ± 0.01 ab | 0.29 ± 0.01 b | 0.32 ± 0.01 a |
| Total caffeoylquinic acids | 0.81 ± 0.04 b | 0.96 ± 0.02 a | 0.93 ± 0.03 a | 0.58 ± 0.01 d | 0.68 ± 0.01 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsivelika, N.; Irakli, M.; Mavromatis, A.; Chatzopoulou, P.; Karioti, A. Phenolic Profile by HPLC-PDA-MS of Greek Chamomile Populations and Commercial Varieties and Their Antioxidant Activity. Foods 2021, 10, 2345. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10102345
Tsivelika N, Irakli M, Mavromatis A, Chatzopoulou P, Karioti A. Phenolic Profile by HPLC-PDA-MS of Greek Chamomile Populations and Commercial Varieties and Their Antioxidant Activity. Foods. 2021; 10(10):2345. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10102345
Chicago/Turabian StyleTsivelika, Nektaria, Maria Irakli, Athanasios Mavromatis, Paschalina Chatzopoulou, and Anastasia Karioti. 2021. "Phenolic Profile by HPLC-PDA-MS of Greek Chamomile Populations and Commercial Varieties and Their Antioxidant Activity" Foods 10, no. 10: 2345. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10102345