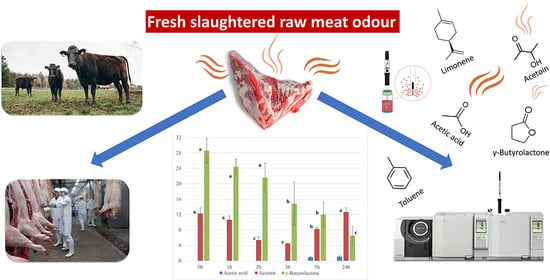
Odor Emissions from Raw Meat of Freshly Slaughtered Cattle during Inspection
Abstract
:1. Introduction
- -
- Which are the volatile compounds emitted by the meat of FSC that could interfere with the possible off-odor perception during the post-mortem inspection?
- -
- How long does this odor last?
2. Materials and Methods
2.1. Sampling
2.2. Volatiles Extraction
2.3. Volatiles Analysis
2.4. Quantitative Analysis
2.5. Method Validation
2.6. Statistical Analysis
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soncini, S.; Chiesa, L.M.; Cantoni, C.; Biondi, P.A. Preliminary study of the volatile fraction in the raw meat of pork, duck and goose. J. Food Compos. Anal. 2007, 20, 436–439. [Google Scholar] [CrossRef]
- Patrizi, F.; Ciani, G. Gli Alimenti di Origine Animale, 1st ed.; Cisalpino: Milano, Italy, 1949. [Google Scholar]
- Caserio, G.; Stecchini, M. Le Carni e i Prodotti Carnei; Clesav: Milano, Italy, 1985. [Google Scholar]
- King, M.F.; Hamilton, B.L.; Matthews, M.A.; Rule, D.C.; Field, R.A. Isolation and identification of volatiles and condensable material in raw beef with supercritical carbon dioxide extraction. J. Agr. Food Chem. 1993, 41, 1974–1981. [Google Scholar] [CrossRef]
- Insausti, K.; Beriain, M.J.; Gorraiz, C.; Purroy, A. Volatile compounds of raw beef from 5 local Spanish cattle breeds stored under modified atmosphere. J. Food Sci. 2002, 67, 1580–1589. [Google Scholar] [CrossRef]
- Perez, R.A.; Rojo, M.D.; Gonzlez, G.; Lorenzo, C.D. Solid-phase microextraction for the determination of volatile compounds in the spoilage of raw ground beef. J. AOAC Int. 2008, 91, 1409–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bueno, M.; Resconi, V.C.; Campo, M.M.; Ferreira, V.; Escudero, A. Development of a robust HS-SPME-GC-MS method for the analysis of solid food samples. Analysis of volatile compounds in fresh raw beef of differing lipid oxidation degrees. Food Chem. 2019, 281, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Lafenêtre, H.; Dedieu, P. Technique Systématique de L’inspection des Viandes de Boucherie, 2nd ed.; Vigot Frères: Paris, France, 1946. [Google Scholar]
- Weir, C.E. Palatability characteristics of meat. In The Science of Meat and Meat Products; W.H. Freemen and Company, American Meat Institute Foundation: San Francisco, CA, USA, 1960; pp. 212–221. [Google Scholar]
- Mantovani, G. Ispezione Degli Alimenti di Origine Animale; UTET: Torino, Italy, 1961; Volume 1. [Google Scholar]
- Wilson, A. Pratica Dell’ispezione Delle Carni, 1st ed.; Marrapese D.E.M.I.: Roma, Italy, 1981. [Google Scholar]
- Romboli, B.; Mantovani, G. Ispezione e Controllo Delle Derrate di Origine Animale; UTET: Torino, Italy, 1985. [Google Scholar]
- Marcato, P.S. Patologia Animale e Ispezione Sanitaria Delle Carni Fresche. Testo e Atlante; Edagricole: Bologna, Italy, 1995. [Google Scholar]
- Guarda, F.; Mandelli, G. Trattato di Anatomia Patologica Veterinaria, 3rd ed.; UTET: Torino, Italy, 2002. [Google Scholar]
- Wallace, H.G.; Weaver, D.B.; Kretzmann, P.M.; Payne, J.R. Bovine parafilariasis: Condemnations at the Cato Ridge abattoir. J. S. Afr. Vet. Assoc. 1983, 54, 123–125. [Google Scholar]
- Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 laying down uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council and amending Commission Regulation (EC) No 2074/2005 as regards official controls. Off. J. Eur. Union 2019, L131, 51–100.
- European Union. Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/ EC and Council Decision 92/438/EEC (Official Controls Regulation). Off. J. Eur. Union 2017, L95, 1–142. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32017R0625 (accessed on 7 October 2021).
- European Union. Regulation EC 2004/853 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. Off. J. Eur. Union 2004, L139, 55–205. [Google Scholar]
- European Commission, Regulation (EU) 2017/1981 of 31 October 2017 amending Annex III to Regulation (EC) No 853/2004 of the European Parliament and of the Council as regards temperature conditions during transport of meat. Off. J. Eur. Union 2017, L285, 10–13.
- Phiri, A.M. Common conditions leading to cattle carcass and offal condemnations at 3 abattoirs in the Western Province of Zambia and their zoonotic implications to consumers. J. S. Afr. Vet. Assoc. 2006, 77, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Alton, G.D.; Pearl, D.L.; Bateman, K.G.; McNab, W.B.; Berke, O. Factors associated with whole carcass condemnation rates in provincially-inspected abattoirs in Ontario 2001-2007: Implications for food animal syndromic surveillance. BMC Vet. Res. 2010, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Jeremiah, L.E.; Gibson, L.L.; Aalhus, J.L.; Dugan, M.E.R. Assessment of palatability attributes of the major beef muscles. Meat Sci. 2003, 65, 949–958. [Google Scholar] [CrossRef]
- Cincotta, F.; Verzera, A.; Tripodi, G.; Condurso, C. Non-intentionally added substances in PET bottled mineral water during the shelf-life. Eur. Food Res. Technol. 2018, 244, 433–439. [Google Scholar] [CrossRef]
- Raboni, M.; Torretta, V.; Viotti, P. Treatment of airborne BTEX by a two-stage biotrickling filter and biofilter, exploiting selected bacterial and fungal consortia. Int. J. Environ. Sci. Technol. 2017, 14, 19–28. [Google Scholar] [CrossRef]
- Boonbumrung, S.; Tamura, H.; Mookdasanit, J.; Nakamoto, H.; Ishihara, M.; Yoshizawa, T.; Varanyanond, W. Characteristic aroma components of the volatile oil of yellow keaw mango fruits determined by limited odor unit method. Food Sci. Technol. Res. 2001, 7, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Cheong, M.W.; Liu, S.Q.; Yeo, J.; Chionh, H.K.; Pramudya, K.; Curran, P.; Yu, B. Identification of aroma-active compounds in Malaysian pomelo (Citrus grandis (L.) Osbeck) peel by gas chromatography-olfactometry. J. Essent. Oil Res. 2011, 23, 34–42. [Google Scholar] [CrossRef]
- Taylor, K.; Wick, C.; Castada, H.; Kent, K.; Harper, W.J. Discrimination of Swiss cheese from 5 different factories by high impact volatile organic compound profiles determined by odor activity value using selected ion flow tube mass spectrometry and odor threshold. J. Food Sci. 2013, 78, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Palomo, E.; Gómez García-Carpintero, E.; Alonso-Villegas, R.; González-Viñas, M.A. Characterization of aroma compounds of Verdejo white wines from the La Mancha region by odour activity values. Flavour Frag. J. 2010, 25, 456–462. [Google Scholar] [CrossRef]
- Qian, M.C.; Wang, Y. Seasonal variation of volatile composition and odor activity value of ‘Marion’ (Rubus spp. hyb) and ‘Thornless Evergreen’ (R. laciniatus L.) blackberries. J. Food Sci. 2005, 70, 13–20. [Google Scholar] [CrossRef]
- Vasta, V.; Priolo, A. Ruminant fat volatiles as affected by diet. A review. Meat Sci. 2006, 73, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Resconi, V.C.; Escudero, A.; Campo, M.M. The development of aromas in ruminant meat. Molecules 2013, 18, 6748–6781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tansawat, R.; Maughan, C.A.; Ward, R.E.; Martini, S.; Cornforth, D.P. Chemical characterisation of pasture-and grain-fed beef related to meat quality and flavour attributes. Int. J. Food Sci. Tech. 2013, 48, 484–495. [Google Scholar] [CrossRef]
- Maruri, J.L.; Larick, D.K. Volatile concentration and flavor of beef as influenced by diet. J. Food Sci. 1992, 57, 1275–1281. [Google Scholar] [CrossRef]
- Lourenço, M.; Ramos-Morales, E.; Wallace, R.J. The role of microbes in rumen lipolysis and biohydrogenation and their manipulation. Animal 2010, 4, 1008–1023. [Google Scholar] [CrossRef] [Green Version]
- Nagaraja, T.G. Microbiology of the Rumen. In Rumenology; Millen, D., De Beni Arrigoni, M., Dias Lauritano Pacheco, R., Eds.; Springer Nature: Basel, Switzerland, 2016; pp. 39–61. [Google Scholar]

| Off-Odor | Origin | References |
|---|---|---|
| Manure | Late-gutted animals | [8] |
| Repugnant | Ingestion of wild garlic, absinthe, fish, rotten meat, rancid oil cake, Greek hay | [8] |
| Renetta apple | Tired animals after transport | [8] |
| Worms | Helminth infections in animals | [8] |
| Fishy | Animals fed ratios high in fish products | [9] |
| Anomalous | Ingestion of ether, chloroform, turpentine essence, chlorine, creolin, camphor, iodine, anise, kummel oil, sulfur, carbolic acid, linseed oil Some substances may be inhaled when stables or trucks are sanitized | [10] |
| Rancid | Protracted feeding of linseed, rapeseed, turnip-seed Fermented or spoiled pumpkins | [10] |
| “Sui generis” | Unripe carobs or residues from industrial processing of carobs Industrial citrus fruit by-products Garlics, onions, crocuses, forage kale | [10] |
| Urinous | Inadequate supply of drinking water | [10] |
| Unpleasant, disgusting fetid | Chrysalis infection | [10] |
| Amniotic fluid | Pregnant cows or that have recently given birth | [11] |
| Cat-like | Anomalous smell acquired during carcass refrigeration Presence of dirt in varnishes, paints, or packaging materials | [11] |
| Urinous | Exhausted animals | [12] |
| Repellent, pig manure-like | Greek hay (Trigonella foenum graecum) ingestion | [12] |
| Anomalous | Inhaled creolin or paint | [12] |
| Anomalous | Ingestion of turnip, silage from poultry litter, spoiled orange, and onion peels | [13] |
| Phosphorus-like | Ingestion of fresh onion leaves | [13] |
| Unpleasant | Ingestion of dieldrin-treated feed | [13] |
| Fecaloid (abdominal muscles and diaphragm) | Delayed evisceration Inadequate bleeding | [10] |
| Something sweet and unpleasant | Cows around the time of parturition | [13] |
| Off-Odor | Origin | References |
|---|---|---|
| Urinous, Ammonia | Uremia | [10] |
| Urinous | Urolithiasis | [10] |
| Fecaloid | Hepatogenic jaundice | [10] |
| Fecaloid | Hepatic pathologies | [10] |
| Putrid | Traumatic pericarditis, with large purulent or ichorous–purulent areas | [10] |
| Ammonia | Traumatic pericarditis | [10] |
| Putrid or putrid and ammonia | Ichorous–purulent peritonitis Sero-fibrinous peritonitis Multiple abscesses within the peritoneal cavity Gangrenous metritis, mastitis, and pneumonia | [10] |
| Peptic | Acute tympany | [10] |
| Fecaloid | Hepatic diseases | [10] |
| Putrid near the utero or in the abdominal regions | Septic metritis and metroperitonitis | [10] |
| Persistent of acetone, coinciding with birth | Ketosis in caws | [10] |
| Repellent and similar to a mixture of ether, ammonia, and methyl alcohol Butyric acid | Calf ascariasis | [10,11,12,13,14] |
| Rancid butter | Blackleg Malignant edema | [10] |
| Anomalous | Bovine slaughtered after a long transport | [10] |
| Cheese-like | Clostridial diseases | [10] |
| Something sweet | Ketosis in caws | [11] |
| Fluid with a rancid smell | Ketosis in caws | [10] |
| Sharp metallic odor (active lesion) | Parafilaria bovicola infection | [15] |
| Anomalous | Jaundice, kidney diseases, placental retention | [12] |
| Something sweet and unpleasant | Fluid build-up in meat, carcasses of feverish animals Ketosis or acetonemia | [13] |
| Unpleasant | Gangrenous injury | [13] |
| Compounds | Precision | Recovery (%) | LOD (µg/g) | LOQ (µg/g) | |
|---|---|---|---|---|---|
| Intraday RSD% | Interday RSD% | ||||
| Toluene | 2.26 | 3.12 | 99.6 | 0.025 | 0.081 |
| Limonene | 3.41 | 4.86 | 99.7 | 0.003 | 0.010 |
| 2-Methyl-3-buten-1-ol | 4.56 | 2.95 | 99.0 | 0.019 | 0.063 |
| p-Cymene | 2.27 | 4.26 | 99.3 | 0.014 | 0.046 |
| Acetoin | 4.96 | 3.71 | 99.1 | 0.038 | 0.123 |
| 6-Methyl-5-hepten-2-one | 5.48 | 5.19 | 99.8 | 0.022 | 0.071 |
| Nonanal | 3.42 | 2.73 | 99.4 | 0.017 | 0.05 |
| Acetic acid | 2.38 | 4.25 | 99.3 | 0.028 | 0.094 |
| γ-Butyrolactone | 5.76 | 3.32 | 99.5 | 0.015 | 0.048 |
| 1-Nonanol | 2.94 | 3.69 | 99.7 | 0.011 | 0.036 |
| Compounds | LRI 1 | Time from Slaughtering/Hours | Odor | OTV 4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 2 | 1 2 | 2 2 | 3 2 | 5 2 | 24 3 | |||||||||||
| X 5 | SD | X | SD | X | SD | X | SD | X | SD | X | SD | ppm | Ref. | |||
| Toluene | 1046 | 0.53 | 0.32 | 0.55 | 0.39 | 0.45 | 0.43 | 0.23 | 0.01 | 0.39 | 0.11 | 0.49 | 0.18 | Sweet, pungent, benzene-like | 1.0–2.9 | [24] |
| Limonene | 1150 | 0.19a 6 | 0.08 | 0.26a | 0.01 | 0.28a | 0.05 | 0.30a | 0.03 | 0.11b | 0.03 | tr c | - | Citrus | 1.2 | [25] |
| 2-Methyl-3-buten-1-ol | 1245 | tr 7 | - | tr | - | tr | - | tr | - | tr | - | tr | - | Butter, sweet, balsamic | 0.25 | [24] |
| p-Cymene | 1287 | tr | - | tr | - | tr | - | tr | - | tr | - | tr | - | Citrus, fresh, solvent | 0.12 | [25] |
| Acetoin | 1294 | 9.83a | 2.40 | 8.52a | 1.28 | 4.32c | 1.39 | 3.61c | 0.30 | 6.61b | 0.30 | 10.15a | 1.72 | Buttery, creamy, dairy, milky, fatty, sweet | 0.8 | [24] |
| 6-Methyl-5-hepten-2-one | 1350 | tr | - | tr | - | tr | - | tr | - | tr | - | tr | - | Citrus, lemon | 1 | [26] |
| Nonanal | 1396 | tr b | - | tr b | - | tr b | - | tr b | - | tr b | - | 0.75 a | 0.20 | Waxy, aldehydic, rose, fresh, orris, orange peel, fatty, peel, green, cucumber | 1 | [24] |
| Acetic acid | 1460 | 0.73b | 0.17 | 0.98b | 0.02 | 0.98b | 0.19 | 0.81b | 0.19 | 1.81a | 0.19 | 2.05a | 0.93 | Pungent, acidic, cheesy, vinegar | 1.958 | [27] |
| γ-Butyrolactone | 1640 | 1.00a | 0.22 | 0.85 | 0.26 | 0.76a | 0.14 | 0.52ab | 0.19 | 0.42b | 0.17 | 0.23b | 0.17 | Sweet, toast, caramel | 0.035 | [28] |
| 1-Nonanol | 1666 | tr | - | tr | - | tr | - | tr | - | tr | - | tr | - | Rose-orange | 1 | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conte, F.; Cincotta, F.; Condurso, C.; Verzera, A.; Panebianco, A. Odor Emissions from Raw Meat of Freshly Slaughtered Cattle during Inspection. Foods 2021, 10, 2411. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10102411
Conte F, Cincotta F, Condurso C, Verzera A, Panebianco A. Odor Emissions from Raw Meat of Freshly Slaughtered Cattle during Inspection. Foods. 2021; 10(10):2411. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10102411
Chicago/Turabian StyleConte, Francesca, Fabrizio Cincotta, Concetta Condurso, Antonella Verzera, and Antonio Panebianco. 2021. "Odor Emissions from Raw Meat of Freshly Slaughtered Cattle during Inspection" Foods 10, no. 10: 2411. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10102411