Latest Advances in Protein-Recovery Technologies from Agricultural Waste
Abstract
:1. Introduction
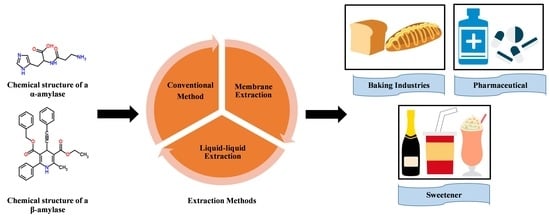
2. Extraction of Amylase from Agricultural Waste
2.1. Characteristics of Agricultural Waste and Protein
2.1.1. Presence of Enzymes in the Agricultural Waste Stream
2.1.2. Classification of Amylase
2.1.3. Presence of Amylase in Agricultural Waste Stream
2.1.4. Application of Amylase
2.2. Conventional Methods in Extraction and Purification of Protein from Agricultural Waste
2.2.1. Membrane Extraction
2.2.2. Precipitation
2.2.3. Ultrasonication
2.2.4. Chromatography
2.2.5. Liquid–Liquid Extraction
2.3. Advanced Liquid–Liquid Extraction Techniques in the Recovery and Purification of Proteins from Agricultural Waste
2.3.1. Liquid Biphasic System
2.3.2. Liquid Biphasic Flotation
2.3.3. Thermoseparation
2.3.4. Three-Phase Partitioning
2.3.5. Integration of LBS with Other Technologies
2.4. Comparison of Conventional and Advanced LLE Techniques
2.5. Parameters in LBS
2.5.1. pH System
2.5.2. Molecular Weight of Polymer
2.5.3. Temperature
2.5.4. Polymer Concentration
2.5.5. Salt Concentration
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Petruccioli, M.; Raviv, M.; Di Silvestro, R.; Dinelli, G. Agriculture and Agro-Industrial Wastes, by-Products, and Wastewaters: Origin, Characteristics, and Potential in Bio-Based Compounds Production, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 6, ISBN 9780444640475. [Google Scholar]
- Obi, F.; Ugwuishiwu, B.; Nwakaire, J. Agricultural waste concept, generation, utilization and management. Niger. J. Technol. 2016, 35, 957. [Google Scholar] [CrossRef]
- Wang, B.; Dong, F.; Chen, M.; Zhu, J.; Tan, J.; Fu, X.; Wang, Y.; Chen, S. Advances in recycling and utilization of agricultural wastes in China: Based on environmental risk, crucial pathways, influencing factors, policy mechanism. Procedia Environ. Sci. 2016, 31, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Azeez, S.; Narayana, C.K.; Oberoi, H.S. Extraction and Utilisation of Bioactive Compounds From Agricultural Waste; Taylor & Francis: Boca Raton, FL, USA, 2017; Chapter 5; ISBN 9781315151540. [Google Scholar]
- Ali, S.; Paul Peter, A.; Chew, K.W.; Munawaroh, H.S.H.; Show, P.L. Resource recovery from industrial effluents through the cultivation of microalgae: A review. Bioresour. Technol. 2021, 125461, 337–348. [Google Scholar] [CrossRef]
- Watford, M.; Wu, G. Protein. Adv. Nutr. 2018, 9, 651–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonnie, M.; Hooker, E.; Brunstrom, J.M.; Corfe, B.M.; Green, M.A.; Watson, A.W.; Williams, E.A.; Stevenson, E.J.; Penson, S.; Johnstone, A.M. Protein for life: Review of optimal protein intake, sustainable dietary sources and the effect on appetite in ageing adults. Nutrients 2018, 10, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, C.Y.; Leong, O.J. Selective recovery of amylase from pitaya (Hylocereus polyrhizus) peel using alcohol/salt aqueous two-phase system. J. Eng. Sci. Technol. 2018, 13, 123–136. [Google Scholar]
- Chaiwut, P.; Pintathong, P.; Rawdkuen, S. Extraction and three-phase partitioning behavior of proteases from papaya peels. Process Biochem. 2010, 45, 1172–1175. [Google Scholar] [CrossRef]
- Ratanapongleka, K. Recovery of biological products in aqueous two phase systems. Int. J. Chem. Eng. Appl. 2010, 1, 191–198. [Google Scholar] [CrossRef]
- Yücekan, I.; Önal, S. Partitioning of invertase from tomato in poly(ethylene glycol)/sodium sulfate aqueous two-phase systems. Process Biochem. 2011, 46, 226–232. [Google Scholar] [CrossRef]
- Bentsen, N.S.; Felby, C.; Thorsen, B.J. Agricultural residue production and potentials for energy and materials services. Prog. Energy Combust. Sci. 2014, 40, 59–73. [Google Scholar] [CrossRef]
- Hicks, T.M.; Verbeek, C.J.R. Protein-Rich By-Products: Production Statistics, Legislative Restrictions, and Management Options; Elsevier: Amsterdam, The Netherlands, 2016; Chapter 1; ISBN 9780128023914. [Google Scholar]
- Shahid, K.; Srivastava, V.; Sillanpää, M. Protein recovery as a resource from waste specifically via membrane technology—from waste to wonder. Environ. Sci. Pollut. Res. 2021, 28, 10262–10282. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Qader, S.A.U.; Aman, A.; Syed, M.N.; Azhar, A. Purification and characterization of novel α-amylase from Bacillus subtilis KIBGE HAS. AAPS PharmSciTech 2011, 12, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Saxena, A.; Tripathi, B.P.; Kumar, M.; Shahi, V.K. Membrane-based techniques for the separation and purification of proteins: An overview. Adv. Colloid Interface Sci. 2009, 145, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Rathnasamy, S.K. β amylase purification from sweet potato (Ipomoea batatus): Reverse micellar extraction versus ammonium sulphate precipitation. Der Pharm. Lett. 2016, 8, 118–125. [Google Scholar]
- Melnichuk, N.; Braia, M.J.; Anselmi, P.A.; Meini, M.R.; Romanini, D. Valorization of two agroindustrial wastes to produce alpha-amylase enzyme from Aspergillus oryzae by solid-state fermentation. Waste Manag. 2020, 106, 155–161. [Google Scholar] [CrossRef]
- De Souza, P.M.; Magalhães, P. de O. Application of microbial α-amylase in industry—A review. Braz. J. Microbiol. 2010, 41, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Gómez-villegas, P.; Vigara, J.; Romero, L.; Gotor, C.; Raposo, S.; Gonçalves, B.; Léon, R. Biochemical characterization of the amylase activity from the new Haloarchaeal strain Haloarcula sp. Hs isolated in the Odiel Marshlands. Biology 2021, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Naidu, M.A.; Saranraj, P. Bacterial Amylase: A Review. Int. J. Pharm. Biol. Arch. 2013, 4, 274–287. [Google Scholar]
- Panesar, P.S.; Kaur, R.; Singla, G.; Sangwan, R.S. Bio-processing of agro-industrial wastes for production of food-grade enzymes: Progress and prospects. Appl. Food Biotechnol. 2016, 3, 208–227. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Suriya, J.; Krishnan, M.; Manivasagan, P.; Kim, S.K. Production of Enzymes from Agricultural Wastes and Their Potential Industrial Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 80. [Google Scholar]
- Nadzirah, K.Z.; Zainal, S.; Noriham, A.; Normah, I. Efficacy of selected purification techniques for bromelain. Int. Food Res. J. 2013, 20, 43–46. [Google Scholar]
- Saini, R.; Singh Saini, H.; Dahiya, A.; Harnek Singh Saini, C. Amylases: Characteristics and industrial applications. J. Pharmacogn. Phytochem. 2017, 6, 1865–1871. [Google Scholar]
- Rana, N.; Walia, A.; Gaur, A. α-amylases from microbial sources and its potential applications in various industries. Natl. Acad. Sci. Lett. 2013, 36, 9–17. [Google Scholar] [CrossRef]
- Sagu, S.T.; Nso, E.J.; Homann, T.; Kapseu, C.; Rawel, H.M. Isolation and purification of beta-amylase from stems of Cadaba farinosa and determination of its characteristics. Curr. Top. Pept. Protein Res. 2016, 17, 21–35. [Google Scholar]
- Sagu, S.T.; Nso, E.J.; Homann, T.; Kapseu, C.; Rawel, H.M. Extraction and purification of beta-amylase from stems of Abrus precatorius by three phase partitioning. Food Chem. 2015, 183, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Telrandhe, U.B.; Uplanchiwar, V. Phyto-Pharmacological perspective of Cadaba farinosa forsk. Am. J. Phytomedicine Clin. Ther. 2018, 1, 11–22. [Google Scholar]
- Amid, M.; Abdul Manap, M.Y.; Zohdi, N. Optimization of processing parameters for extraction of amylase enzyme from dragon (Hylocereus polyrhizus) peel using response surface methodology. Sci. World J. 2014, 2014, 640949. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.; Lin, C.G.; Chen, W.L.; Huang, Y.C.; Chen, C.Y.; Huang, K.F.; Yang, C.H. Evaluation of the antioxidant and wound-healing properties of extracts from different parts of Hylocereus polyrhizus. Agronomy 2019, 9, 27. [Google Scholar] [CrossRef] [Green Version]
- Lawal, A.K.; Banjoko, A.M.; Olatope, S.O.; Alebiosu, F.A.; Orji, F.A.; Suberu, Y.L.; Itoandon, E.E.; Shittu, K.A.; Adelaja, O.D.; Ojo, E.; et al. Production and partial purification of amylase by Aspergillus niger isolated from cassava peel. J. Basic Appl. Sci. 2014, 10, 287–291. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Avramescu, S.M.; Sieniawska, E. Recovery of natural antioxidants from agro-industrial side streams through advanced extraction techniques. Molecules 2019, 24, 4212. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.E.; Franco, T.T. Liquid-liquid extraction of biomolecules in downstream processing—A review paper. Braz. J. Chem. Eng. 2000, 17, 1–17. [Google Scholar] [CrossRef]
- Vithu, P.; Dash, S.K.; Rayaguru, K. Post-harvest processing and utilization of sweet potato: A review. Food Rev. Int. 2019, 35, 726–762. [Google Scholar] [CrossRef]
- Brojanigo, S.; Parro, E.; Cazzorla, T.; Favaro, L.; Basaglia, M.; Casella, S. Conversion of starchy waste streams into polyhydroxyalkanoates using Cupriavidus necator DSM 545. Polymers 2020, 12, 1496. [Google Scholar] [CrossRef]
- He, C.; Sampers, I.; Raes, K. Dietary fiber concentrates recovered from agro-industrial by-products: Functional properties and application as physical carriers for probiotics. Food Hydrocoll. 2021, 111, 106175. [Google Scholar] [CrossRef]
- Jönsson, J.Å. Membrane-based extraction for environmental analysis. Compr. Sampl. Sample Prep. 2012, 3, 591–602. [Google Scholar] [CrossRef]
- Jain, S.; Anal, A.K. Optimization of extraction of functional protein hydrolysates from chicken egg shell membrane (ESM) by ultrasonic assisted extraction (UAE) and enzymatic hydrolysis. LWT-Food Sci. Technol. 2016, 69, 295–302. [Google Scholar] [CrossRef]
- Rodrigues, É.F.; Ficanha, A.M.M.; Dallago, R.M.; Treichel, H.; Reinehr, C.O.; Machado, T.P.; Nunes, G.B.; Colla, L.M. Production and purification of amylolytic enzymes for saccharification of microalgal biomass. Bioresour. Technol. 2017, 225, 134–141. [Google Scholar] [CrossRef]
- Nor, M.Z.M.; Ramchandran, L.; Duke, M.; Vasiljevic, T. Performance of a two-stage membrane system for bromelain separation from pineapple waste mixture as impacted by enzymatic pretreatment and diafiltration. Food Technol. Biotechnol. 2018, 56, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.R.; Severo, E.E.; de Oliveira, J.M.; Ores, J.D.C.; Brandelli, A.; Kalil, S.J. One-step ultrafiltration process for separation and purification of a keratinolytic protease produced with feather meal. Int. J. Chem. Eng. 2018, 2018, 6729490. [Google Scholar] [CrossRef]
- Parhi, P.K. Supported liquid membrane principle and its practices: A short review. J. Chem. 2013, 2013, 618236. [Google Scholar] [CrossRef]
- Eljaddi, T.; Lebrun, L.; Hlaibi, M. Review on mechanism of facilitated transport on liquid membranes. J. Membr. Sci. Res. 2017, 3, 199–208. [Google Scholar] [CrossRef]
- Van Alstine, J.M.; Jagschies, G.; Łacki, K.M. Alternative separation methods: Flocculation and precipitation. Biopharm. Process. Dev. Des. Implement. Manuf. Process. 2018, 221–239. [Google Scholar] [CrossRef]
- Rajalingam, D.; Loftis, C.; Xu, J.J.; Kumar, T.K.S. Trichloroacetic acid-induced protein precipitation involves the reversible association of a stable partially structured intermediate. Protein Sci. 2009, 18, 980–993. [Google Scholar] [CrossRef] [Green Version]
- Haslaniza, H.; Maskat, M.Y.; Wan Aida, W.M.; Mamot, S. Process development for the production of protein hydrolysate from cockle (Anadara granosa) meat wash water. Sains Malays. 2014, 43, 53–63. [Google Scholar]
- Thippeswamy, S.; Girigowda, K.; Mulimani, V.H. Isolation and identification of α-amylase producing Bacillus sp. from dhal industry waste. Indian J. Biochem. Biophys. 2006, 43, 295–298. [Google Scholar]
- Zusfahair, Z.; Ningsih, D.R.; Kartika, D.; Fatoni, A. Amylase from Bacillus thuringiensis isolated from tapioca waste: Isolation, partial purification and characterization. Malays. J. Fundam. Appl. Sci. 2016, 12, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Chemat, F.; Rombaut, N.; Sicaire, A.; Meullemiestre, A.; Abert-vian, M. Ultrasonics sonochemistry ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lamsal, B.P. Ultrasound-assisted extraction and modification of plant-based proteins: Impact on physicochemical, functional, and nutritional properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1457–1480. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.Y.; Liu, L.N.; Xie, Y.P.; Ke, Y.J.; Cai, Z.J.; Wu, G.J. Ultrasonic-assisted extraction and functional properties of wampee seed protein. Food Sci. Technol. 2019, 39, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Ramachandraiah, K.; Jiang, G.; Eun, J.B. Effects of ultra-sonication and agitation on bioactive. Foods 2020, 9, 1116. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Dey, S.; Sen, R. Betalains from Amaranthus tricolor L. J. Pharmacogn. Phytochem. 2013, 32 (Suppl. 1), 71–84. [Google Scholar]
- Vilaplana, F.; Karlsson, P.; Ribes-Greus, A.; Ivarsson, P.; Karlsson, S. Analysis of brominated flame retardants in styrenic polymers. Comparison of the extraction efficiency of ultrasonication, microwave-assisted extraction and pressurised liquid extraction. J. Chromatogr. A 2008, 1196–1197, 139–146. [Google Scholar] [CrossRef]
- Coskun, O. Separation Tecniques: Chromatography. North. Clin. Istanbul 2016, 3, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.; Mestmäcker, F.; Brückner, L.; Elwert, T.; Strube, J. Liquid-liquid extraction and chromatography process routes for the purification of lithium. Mater. Sci. Forum 2019, 959, 79–99. [Google Scholar] [CrossRef] [Green Version]
- Klett, J. Structural motifs of alkali metal superbases in non-coordinating solvents. Chem.-Eur. J. 2021, 27, 888–904. [Google Scholar] [CrossRef]
- Mazzola, P.; Lopes, M.A.; Hasmann, F.A.; Jozala, A.F.; Penna, T.C.; Magalhaes, P.O.; Rangel-Yagui, C.O.; Jr, A.P. Review liquid–liquid extraction of biomolecules: An overview and update of the main techniques. J. Chem. Technol. Biotechnol. 2008, 83, 1163–1169. [Google Scholar] [CrossRef]
- Pereira, J.F.B.; Coutinho, J.A.P. Aqueous Two-Phase Systems; Elsevier: Detroit, MI, USA, 2020; Chapter 5; ISBN 9780128169117. [Google Scholar]
- Daso, A.P.; Okonkwo, O.J. Conventional extraction techniques: Soxhlet and liquid-liquid extractions and evaporation. Anal. Sep. Sci. 2015, 5, 1437–1468. [Google Scholar]
- Berk, Z. Extraction; Academic Press: San Diego, CA, USA, 2018; ISBN 9780128120187. [Google Scholar]
- Simon, L. Aqueous two-phase extraction—A case study in process analysis and control. In Proceedings of the AIChE Annual Meeting, Cincinatti, OH, USA, 30 October–4 November 2005; p. 3777. [Google Scholar]
- Shad, Z.; Mirhosseini, H.; Hussin, A.S.M.; Forghani, B.; Motshakeri, M.; Manap, M.Y.A. Aqueous two-phase purification of α-amylase from white pitaya (Hylocereus undatus) peel in polyethylene glycol/citrate system: Optimization by response surface methodology. Biocatal. Agric. Biotechnol. 2018, 14, 305–313. [Google Scholar] [CrossRef]
- Saw, H.S.; Sankaran, R.; Khoo, K.S.; Chew, K.W.; Phong, W.N.; Tang, M.S.Y.; Lim, S.S.; Mohd Zaid, H.F.; Naushad, M.; Show, P.L. Application of a liquid biphasic flotation (LBF) system for protein extraction from Persiscaria tenulla leaf. Processes 2020, 8, 247. [Google Scholar] [CrossRef] [Green Version]
- Ketnawa, S.; Rungraeng, N.; Rawdkuen, S. Phase partitioning for enzyme separation: An overview and recent applications. Int. Food Res. J. 2017, 24, 1–24. [Google Scholar]
- Hong Yang, A.M.G. Aqueous two-phase extraction advances for bioseparation. J. Bioprocess. Biotech. 2013, 4, 140. [Google Scholar] [CrossRef] [Green Version]
- Zhi, W.; Song, J.; Ouyang, F.; Bi, J. Application of response surface methodology to the modeling of α-amylase purification by aqueous two-phase systems. J. Biotechnol. 2005, 118, 157–165. [Google Scholar] [CrossRef]
- Babu, B.R.; Rastogi, N.K.; Raghavarao, K.S.M.S. Liquid-liquid extraction of bromelain and polyphenol oxidase using aqueous two-phase system. Chem. Eng. Process. Process Intensif. 2008, 47, 83–89. [Google Scholar] [CrossRef]
- Leong, H.Y.; Ooi, C.W.; Law, C.L.; Julkifle, A.L.; Ling, T.C.; Show, P.L. Application of liquid biphasic flotation for betacyanins extraction from peel and flesh of Hylocereus polyrhizus and antioxidant activity evaluation. Sep. Purif. Technol. 2018, 201, 156–166. [Google Scholar] [CrossRef]
- Tham, P.E.; Ng, Y.J.; Sankaran, R.; Khoo, K.S.; Chew, K.W.; Yap, Y.J.; Malahubban, M.; Zakry, F.A.A.; Show, P.L. Recovery of protein from dairy milk waste product using alcohol-salt liquid biphasic flotation. Processes 2019, 7, 875. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Na, J.; Wang, L.; Li, D.; Liu, C.; Feng, Z. Reutilization of food waste: One-step extration, purification and characterization of ovalbumin from salted egg white by aqueous two-phase flotation. Foods 2019, 8, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankaran, R.; Loke Show, P.; Jiun Yap, Y. Liquid biphasic flotation (Lbf) system: Integration of fermentation and recovery process of lipase from Burkholderia Cepacia. In Proceedings of the 72nd IASTEM International Conference, Melbourne, Australia, 5–6 September 2017; pp. 32–36. [Google Scholar]
- Leong, Y.K.; Show, P.L.; Lan, J.C.W.; Loh, H.S. Thermoseparating aqueous two-phase system: Recent trends and applications. Chem. Eng. Trans. 2015, 45, 1249–1254. [Google Scholar] [CrossRef]
- Ketnawa, S.; Benjakul, S.; Martínez-Alvarez, O.; Rawdkuen, S. Thermoseparating aqueous two-phase system for the separation of alkaline proteases from fish viscera. Sep. Sci. Technol. 2014, 49, 2158–2168. [Google Scholar] [CrossRef] [Green Version]
- Show, P.L.; Tan, C.P.; Shamsul Anuar, M.; Ariff, A.; Yusof, Y.A.; Chen, S.K.; Ling, T.C. Extractive fermentation for improved production and recovery of lipase derived from Burkholderia cepacia using a thermoseparating polymer in aqueous two-phase systems. Bioresour. Technol. 2012, 116, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Wang, Y.Y.; Qiu, W.Y.; Ma, H.; Wang, Z.B.; Wu, J.Y. Three-phase partitioning as an elegant and versatile platform applied to nonchromatographic bioseparation processes. Crit. Rev. Food Sci. Nutr. 2018, 58, 2416–2431. [Google Scholar] [CrossRef]
- Gagaoua, M.; Hafid, K. Three phase partitioning system, an emerging non-chromatographic tool for proteolytic enzymes recovery and purification. Biosens. J. 2016, 5, 135–139. [Google Scholar] [CrossRef]
- Chew, K.W.; Ling, T.C.; Show, P.L. Recent developments and applications of three-phase partitioning for the recovery of proteins. Sep. Purif. Rev. 2019, 48, 52–64. [Google Scholar] [CrossRef]
- Vasudev, H.; Singh, G.; Bansal, A.; Vardhan, S.; Thakur, L. Microwave heating and its applications in surface engineering: A review. Smart Mater. Struct. 2018, 6, 102001. [Google Scholar] [CrossRef]
- Xie, X.; Zhu, D.; Zhang, W.; Huai, W.; Wang, K.; Huang, X.; Zhou, L.; Fan, H. Microwave-assisted aqueous two-phase extraction coupled with high performance liquid chromatography for simultaneous extraction and determination of four flavonoids in Crotalaria sessiliflora L. Ind. Crops Prod. 2017, 95, 632–642. [Google Scholar] [CrossRef]
- Wang, H.; Dong, Y.; Xiu, Z.L. Microwave-assisted aqueous two-phase extraction of piceid, resveratrol and emodin from Polygonum cuspidatum by ethanol/ammonium sulphate systems. Biotechnol. Lett. 2008, 30, 2079–2084. [Google Scholar] [CrossRef]
- Phongthai, S.; Lim, S.T.; Rawdkuen, S. Optimization of microwave-assisted extraction of rice bran protein and its hydrolysates properties. J. Cereal Sci. 2016, 70, 146–154. [Google Scholar] [CrossRef]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason. Sonochem. 2013, 20, 1076–1080. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chew, K.W.; Yew, G.Y.; Manickam, S.; Ooi, C.W.; Show, P.L. Integrated ultrasound-assisted liquid biphasic flotation for efficient extraction of astaxanthin from Haematococcus pluvialis. Ultrason. Sonochem. 2020, 67, 105052. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, T.; Antov, M. Ultrasound assisted extraction in aqueous two-phase system for the integrated extraction and separation of antioxidant. Sep. Purif. Technol. 2020. [Google Scholar] [CrossRef]
- Kingwascharapong, P.; Chaijan, M.; Karnjanapratum, S. Ultrasound-assisted extraction of protein from bombay locusts and its impact on functional and antioxidative properties. Sci. Rep. 2021, 11, 17320. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.Y.; Chang, Y.K.; Ooi, C.W.; Law, C.L.; Julkifle, A.L.; Show, P.L. Liquid biphasic electric partitioning system as a novel integration process for betacyanins extraction from red-purple pitaya and antioxidant properties assessment. Front. Chem. 2019, 7, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Khoo, K.S.; Leong, H.Y.; Chew, K.W.; Lim, J.W.; Ling, T.C.; Show, P.L.; Yen, H.W. Liquid biphasic system: A recent bioseparation technology. Processes 2020, 8, 149. [Google Scholar] [CrossRef] [Green Version]
- Scherer, D.; Krust, D.; Frey, W.; Mueller, G.; Nick, P.; Gusbeth, C. Pulsed electric field (PEF)-assisted protein recovery from Chlorella vulgaris is mediated by an enzymatic process after cell death. Algal Res. 2019, 41, 101536. [Google Scholar] [CrossRef]
- Varghese, T.; Pare, A. Effect of microwave assisted extraction on yield and protein characteristics of soymilk. J. Food Eng. 2019, 262, 92–99. [Google Scholar] [CrossRef]
- Biswas, B.; Sit, N. Effect of ultrasonication on functional properties of tamarind seed protein isolates. J. Food Sci. Technol. 2020, 57, 2070–2078. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; You, L.; Dou, W.; Sun, T.; Xu, P. Effects of an electric field on the conformational transition of the protein: A molecular dynamics simulation study. Polymers 2019, 11, 282. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B.; et al. Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Proced. Online 2016, 18, 18. [Google Scholar] [CrossRef] [Green Version]
- Jaffer, Z.M.; Hameed, K.W.; Imran, S.G. Extraction of prodigiosin using aqueous two phase system. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Diyala, Iraq, 16–17 December 2020; Volume 1076, p. 012026. [Google Scholar]
- Asenjo, J.A.; Andrews, B.A. Aqueous two-phase systems for protein separation: Phase separation and applications. J. Chromatogr. A 2012, 1238, 1–10. [Google Scholar] [CrossRef]
- Hatti-Kaul, R. Aqueous Two-Phase Systems. Mol. Biotechnol. 2001, 19, 271–277. [Google Scholar] [CrossRef]
- Grilo, A.L.; Aires-Barros, M.R.; Azevedo, A.M. Partitioning in aqueous two-phase systems: Fundamentals, applications and trends. Sep. Purif. Rev. 2016, 45, 68–80. [Google Scholar] [CrossRef]
- Raja, S.; Murty, V.R. Optimization of aqueous two-phase systems for the recovery of soluble proteins from tannery wastewater using response surface methodology. J. Eng. 2013, 2013, 217483. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, A.; Sen, K. Impact of pH and temperature on phase diagrams of different aqueous biphasic systems. J. Chromatogr. A 2016, 1433, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.A.; Teixeira, J.A. Salt effect on the aqueous two-phase system PEG 8000-sodium sulfate. J. Chem. Eng. Data 2011, 56, 133–137. [Google Scholar] [CrossRef] [Green Version]



| Classification of Amylases | Alternative Names | Applications |
|---|---|---|
| Alpha-amylase | α-1,4-glucan-glucanohydrolase; EC 3.2.1.1 | Degrades the α-1,4-glycosidic linkage of starch by breaking down starch to oligosaccharides or saccharides |
| Beta-amylase | 1,4-D-glucan maltohydrolase; glycogenase; saccharogen amylase; EC 3.2.1.2 | Catalyzes the hydrolysis of the second α-1,4-glycosidic linkage by cleaving the linkage from the non-reducing end |
| Gamma-amylase | Glucan-1,4-α-glucosidase; amyloglucosidase; exo-1,4-α-glucosidase; glucoamylase; lysosomal α-glucosidase; 1,4-α-D-glucan glucohydrolase | Breaks the α-1,6-glycosidic linkage and the last α-1,4-glycosidic linkage at the non-reducing end of amylopectin and amylose |
| Criteria | Conventional LLE Methods | Advanced LLE Methods |
|---|---|---|
| Impact on the Environment | Requires the use of large volumes of highly pure solvents, where the reagents employed are no longer usable or recyclable, and thus, must be dumped into the environment, resulting in negative consequences to the environment | Phase-forming components are non-toxic and environmentally friendly compared to conventional solvents |
| Process Feasibility | Recovery and extraction involve several steps, intricate routes, longer processing time, substantial energy inputs, and are also costly | Simpler and faster, equilibrium distribution takes place in a short time, with low cost and the possibility to be applied in a large-scale separation process |
| Separation Efficiency | The development of an emulsion during the extraction of a specific aqueous sample is due to the presence of surface-active chemicals on some natural materials. These surface-active compounds will adsorb at the liquid–liquid interface, resulting in the formation of an emulsion | The phase-forming components in the advanced LLE method comprise a considerable volume of water while maintaining a low interfacial layer between the two phases |
| Interfacial Tension | High interfacial tension between 1 and 20 dyne/cm for a conventional water–organic solvent system | Low interfacial tension between 0.0001 and 0.1 dyne/cm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusree, F.I.F.M.; Peter, A.P.; Mohd Nor, M.Z.; Show, P.L.; Mokhtar, M.N. Latest Advances in Protein-Recovery Technologies from Agricultural Waste. Foods 2021, 10, 2748. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10112748
Yusree FIFM, Peter AP, Mohd Nor MZ, Show PL, Mokhtar MN. Latest Advances in Protein-Recovery Technologies from Agricultural Waste. Foods. 2021; 10(11):2748. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10112748
Chicago/Turabian StyleYusree, Farhana Iylia Fatinee Mohd, Angela Paul Peter, Mohd Zuhair Mohd Nor, Pau Loke Show, and Mohd Noriznan Mokhtar. 2021. "Latest Advances in Protein-Recovery Technologies from Agricultural Waste" Foods 10, no. 11: 2748. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10112748