A Physiological-Based Model for Simulating the Bioavailability and Kinetics of Sulforaphane from Broccoli Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pre-Modeling Data Processing
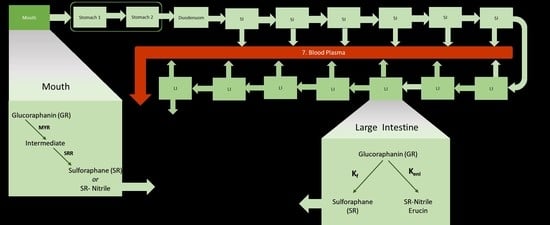
2.2. Model Description and Assumptions
| Broccoli Products Composition | ||||
|---|---|---|---|---|
| Product | Myrosinase Content (mg MYR/mg Broccoli) | Initial GR Concentration (µM) | Initial SR Concentration (µM) | Reference |
| HighBP | 3.49 × 10−2 | 383.3 | 354.2 | [35,42] |
| HighBF | 3.49 × 10−2 | 621.9 | 115.6 | [35,42] |
| MedBF | 6.53 × 10−3 | 667.7 | 69.8 † | [35,42] |
| LowBF | 5.63 × 10−4 | 708.3 | 29.2 † | [35,42] |
| NoBF | 1.13 × 10−5 | 734.4 | 3.1 | [35,42] |
| Oral GR Conversion | ||||
| MMSI Vmax for glucoraphanin | 2070 µmol/min | [42] | ||
| MMSI Km for glucoraphanin | 110.2 µM | [42] | ||
| MMSI Ki for glucoraphanin | 893.0 µM | [42] | ||
| Amount of broccoli in broccoli product | 5000 mg | [35] | ||
| Fraction of sulforaphane converted from GR in mouth | Estimated | |||
| Gastro-Intestinal | ||||
| Mouth to Stomach rate constant | 30 min−1 (60 min−1 to 1 min−1) * | [43,44] | ||
| Stomach emptying time | 30 min | |||
| Gastric rate constant from 2nd stomach to duodenum | S = −ln(0.05)/St | [45] | ||
| Rate constant from 1st to 2nd stomach compartment | Estimated ** | |||
| Small Intestine transit rate constant | Estimated ** | |||
| Large Intestine transit rate constant | Estimated ** | |||
| Absorption rate constant | 0.180 min−1 | [41] | ||
| Elimination rate constant of ITC and ITC conjugates from blood | Estimated ** | |||
| Number of SI compartments (excluding duodenum) | 6 | [26] | ||
| Number of LI compartments | 7 | [26] | ||
| Product + Saliva | 0.096 L (0.095–0.098 L) * | [35,38] | ||
| Stomach: + Raisin bun + gastric secretions | 0.2 L (0.167–0.253 L) * | [35,46] | ||
| Stomach 1 | 0.05 L | |||
| Stomach 2 | 0.15 L | |||
| + duodenal secretions | 0.2 L (0.246–0.332 L) * | [46] | ||
| SI volume (excluding duodenum) | 1.5 L (0.638–1.963 L) * | [47] | ||
| = SI compartment volume | 0.25 L | |||
| LI volume | 3.4 L (3.347–3.492 L) * | [47] | ||
| = LI compartment volume | 0.5 L | |||
| Blood volume of adult | 5 L (4–6 L) * | [48] | ||
| Gut GR Conversion | ||||
| Microbial ITC formation rate constant | Estimated | |||
| GR to erucin and nitriles | Estimated | |||
2.3. Compartmental Mathematics
2.4. Simulink Model
2.5. Matlab Coding and Fittings
2.6. Statistical and Data Analysis
3. Results
3.1. Sensitivity Analysis and Parameters Selection
3.2. Model Fittings
3.3. Bioavailability of Sulforaphane
3.4. Mouth and Gut Parameter Estimations
3.5. Certainty of Parameter Estimates
3.6. Goodness of Fit
4. Discussion
4.1. Sensitivity Analysis and Selection of Parameters
4.2. Model Fittings
4.3. Bioavailability of Sulforaphane
4.4. Mouth and Gut Parameter Estimations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Myrosinase Calculations
- 1.
- Experimental data points were extracted from the reaction rate vs. sinigrin concentration graph [42].
| Sinigrin Concentration (µM) | Initial Reaction Rate (µmol/min) |
| 5 | 0.03 |
| 10 | 0.05 |
| 25 | 0.16 |
| 40 | 0.24 |
| 50 | 0.29 |
| 75 | 0.42 |
| 100 | 0.66 |
| 149 | 0.58 |
| 199 | 0.59 |
| 248 | 0.57 |
| 298 | 0.52 |
- 2.
- The MMSI equation (Equation (A1)) was used to model the data. The parameters, Vmax, Km, and Ki, were solved by minimizing the sum of squares difference using Excel’s SUMXMY2 function and solver.
| Parameter Estimates | |
| Vmax | 0.96 µM/min |
| Km | 86.51 µM |
| Ki | 780.05 µM |
- 3.
- The specific enzyme activity of Myrosinase was calculated using information from the materials Oliviero et al. used to determine activity and the parameter estimates from step 2.
- 4.
- The Oliviero MYR activity (column A below) for each product type was divided by 5000mg to determine the µmol MYR/mg broccoli * min (column B). Column B was divided by the specific enzyme activity (0.178 µmol/mg MYR*min) to obtain the mg MYR/mg Broccoli (column C).
| A | B | C | |
| MYR Activity (Units/5 g dry wt Broccoli) | µmol/mg Broccoli * min | mg MYR/mg Broccoli | |
| High MYR BP | 31 | 0.0062 | 0.035 |
| High MYR BF | 31 | 0.0062 | 0.035 |
| Medium MYR BF | 5.8 | 0.00116 | 0.007 |
| Low MYR BF | 0.5 | 0.0001 | 0.000563 |
| No MYR BF (<0.01) | 0.01 | 0.000002 | 0.0000113 |
- 1.
- Experimental data points were extracted from the reaction rate vs. glucoraphanin concentration graph [42].
| Glucoraphanin Concentration (µM) | Initial Reaction Rate (µmol/min) |
| 5 | 0.02 |
| 8 | 0.09 |
| 10 | 0.11 |
| 25 | 0.25 |
| 50 | 0.45 |
| 75 | 0.7 |
| 90 | 1.14 |
| 100 | 1.33 |
| 150 | 1.25 |
| 200 | 1.19 |
| 250 | 1.18 |
| 300 | 1.05 |
- 2.
- The MMSI equation (Equation (A1)) was used to model the data. The parameters, Vmax, Km, and Ki, were solved by minimizing the sum of squares difference using Excel’s SUMXMY2 function and solver.
| Parameter Estimates | |
| Vmax | 2070 µmol/min |
| Km | 110.16 µM |
| Ki | 893.02 µM |
Appendix B. Parameter Estimates Results and Discussion for KSH, KtSI, KtLI, and Ke
| High BP(n = 15) * | High BF(n = 15) * | Med BF(n = 14) * | Low BF(n = 14) | No BF(n = 14) |
|---|---|---|---|---|
 |  |  |  |  |
| Median: 0.009 min−1 IQR: 0.008 min−1 IQR/Med: 0.9 Outliers: d (9.408 min−1) l (0.752 min−1) p (0.025min−1) | Median: 0.007 min−1 IQR: 0.004 min−1 IQR/Med: 0.6 Outliers: d (0.105 min−1) | Median: 0.017 min−1 IQR: 0.014 min−1 IQR/Med: 0.8 Outliers: d (0.181 min−1) | Median: 0.016 min−1 IQR: 0.014 min−1 IQR/Med: 0.9 Outliers: None | Median: 0.014 min−1 IQR: 0.011 min−1 IQR/Med: 0.8 Outliers: None |
| High BP(n = 15) * | High BF(n = 15) * | Med BF(n = 14) * | Low BF(n = 14) | No BF(n = 14) |
|---|---|---|---|---|
 |  |  |  |  |
| Median: 0.020 min−1 IQR: 0.098 min−1 IQR/Med: 4.9 Outliers: c (5.192 min−1) | Median: 0.023 min−1 IQR: 0.057 min−1 IQR/Med: 2.5 Outliers: n (1.369 min−1) | Median: 0.023 min−1 IQR: 0.014 min−1 IQR/Med: 0.6 Outliers: h (0.366 min−1) i (0.092 min−1) o (0.054 min−1) | Median: 0.020 min−1 IQR: 0.013 min−1 IQR/Med: 0.7 Outliers: none | Median: 0.026 min−1 IQR: 0.009 min−1 IQR/Med: 0.3 Outliers: none |
| High BP(n = 15) | High BF(n = 15) | Med BF(n = 14) * | Low BF(n = 14) * | No BF(n = 14) * |
|---|---|---|---|---|
| ---- | ---- |  |  |  |
| Not fitted. KtLI fixed at 0.003 min−1. | Not fitted. KtLI fixed at 0.003 min−1. | Median: 0.280 min−1 IQR: 0.284 min−1 IQR/Med: 1.0 Outliers: h (0.96575 min−1) i (0.92453 min−1) | Median: 0.220 min−1 IQR: 0.139 min−1 IQR/Med: 0.6 Outliers: n (0.9998 min−1) | Median: 0.031 min−1 IQR: 0.175 min−1 IQR/Med: 5.6 Outliers: q (1.0385 min−1) |
| High BP(n = 15) | High BF(n = 15) | Med BF(n = 14) * | Low BF(n = 14) | No B(n = 14) * |
|---|---|---|---|---|
| ---- | ---- |  |  |  |
| Not fitted. ke fixed at 0.024 min−1. | Not fitted. ke fixed at 0.024 min−1. | Median: 0.020 min−1 IQR: 0.043 min−1 IQR/Med: 2.2 Outliers: c (0.277 min−1) g (0.319 min−1) o (0.295 min−1) | Median: 0.025 min−1 IQR: 0.028 min−1 IQR/Med: 1.1 Outliers: None | Median: 0.017 min−1 IQR: 0.01 min−1 IQR/Med: 0.6 Outliers: f (0.038 min−1) g (0.039 min−1) |
References
- Mérillon, J.M.; Ramawat, K.G. Glucosinolates; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, T.; Verkerk, R.; Dekker, M. Isothiocyanates from brassica Vegetables-Effects of processing, cooking, mastication, and digestion. Mol. Nutr. Food Res. 2018, 62, e1701069. [Google Scholar] [CrossRef] [Green Version]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; De Schrijver, R.; Hansen, M.; Gerhäuser, C.; Mithen, R.; et al. Glucosinolates in brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53, S219. [Google Scholar] [CrossRef]
- Nugrahedi, P.Y.; Verkerk, R.; Widianarko, B.; Dekker, M. A mechanistic perspective on Process-Induced changes in glucosinolate content in brassica vegetables: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 823–838. [Google Scholar] [CrossRef]
- Saha, S.; Hollands, W.; Teucher, B.; Needs, P.W.; Narbad, A.; Ortori, C.A.; Barrett, D.A.; Rossiter, J.T.; Mithen, R.F.; Kroon, P.A. Isothiocyanate concentrations and interconversion of sulforaphane to erucin in human subjects after consumption of commercial frozen broccoli compared to fresh broccoli. Mol. Nutr. Food Res. 2012, 56, 1906–1916. [Google Scholar] [CrossRef]
- Li, F.; Hullar, M.A.J.; Beresford, S.A.A.; Lampe, J.W. Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. Br. J. Nutr. 2011, 106, 408–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullaney, J.A.; Kelly, W.J.; McGhie, T.K.; Ansell, J.; Heyes, J.A. lactic acid bacteria convert glucosinolates to nitriles efficiently yet differently from enterobacteriaceae. J. Agric. Food Chem. 2013, 61, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Narbad, A.; Rossiter, J.T. Gut glucosinolate metabolism and isothiocyanate production. Mol. Nutr. Food Res. 2018, 62, e1700991. [Google Scholar] [CrossRef]
- Palop, M.L.; Smiths, J.P.; ten Brink, B. Degradation of sinigrin by Lactobacillus agilis strain R16. Int. J. Food Microbiol. 1995, 26, 219–229. [Google Scholar] [CrossRef]
- Rabot, S.; Guerin, C.; Nugon-Baudon, L.; Szylit, O. Glucosinolate degradation by bacterial strains isolated from a human intestinal microflora. In 9th International Rapeseed Congress; GCIRC: Cambridge, UK; Paris, France, 1995. [Google Scholar]
- Elfoul, L.; Rabot, S.; Khelifa, N.; Quinsac, A.; Duguay, A.; Rimbault, A. Formation of allyl isothiocyanate from sinigrin in the digestive tract of rats monoassociated with a human colonic strain of Bacteroides thetaiotaomicron. FEMS Microbiol. Lett. 2001, 197, 99–103. [Google Scholar] [CrossRef]
- Luang-In, V.; Narbad, A.; Nueno-Palop, C.; Mithen, R.; Bennett, M.; Rossiter, J.T. The metabolism of methylsulfinylalkyl- and methylthioalkyl-glucosinolates by a selection of human gut bacteria. Mol. Nutr. Food Res. 2014, 58, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.L.; Hashimoto, K.; Uda, Y. In vitro digestion of sinigrin and glucotropaeolin by single strains of Bifidobacterium and identification of the digestive products. Food Chem. Toxicol. 2004, 42, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Kühn, C.; Kupke, F.; Baldermann, S.; Klopsch, R.; Lamy, E.; Hornemann, S.; Pfeiffer, A.F.H.; Schreiner, M.; Hanschen, F.S.; Rohn, S. Diverse excretion pathways of benzyl glucosinolate in humans after consumption of nasturtium (Tropaeolum majus L.)—A pilot study. Mol. Nutr. Food Res. 2018, 62, 1800588. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; da Silva Pinto, M. Improvement of bioaccessibility and bioavailability: From molecular interactions to delivery systems. In Engineering Foods for Bioactives Stability and Delivery; Roos, Y.H., Livney, Y.D., Eds.; Springer: New York, NY, USA, 2017; pp. 401–416. [Google Scholar]
- Capuano, E.; Pellegrini, N. An integrated look at the effect of structure on nutrient bioavailability in plant foods. J. Sci. Food Agric. 2019, 99, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, J.M. The food matrix: Implications in processing, nutrition and health. Crit. Rev. Food Sci. Nutr. 2019, 59, 3612–3629. [Google Scholar] [CrossRef]
- Sarvan-Kruse, I.; Kramer, E.; Bouwmeester, H.; Dekker, M.; Verkerk, R. Sulforaphane formation and bioaccessibility are more affected by steaming time than meal composition during in vitro digestion of broccoli. Food Chem. 2017, 214, 580–586. [Google Scholar] [CrossRef]
- Sarvan, I.; van der Klauw, M.; Oliviero, T.; Dekker, M.; Verkerk, R. The effect of chewing on oral glucoraphanin hydrolysis in raw and steamed broccoli. J. Funct. Foods 2018, 45, 306–312. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Kühn, C.; Nickel, M.; Rohn, S.; Dekker, M. Leaching and degradation kinetics of glucosinolates during boiling of Brassica oleracea vegetables and the formation of their breakdown products. Food Chem. 2018, 263, 240–250. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Juvik, J.A.; Jeffery, E.H. Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry 2004, 65, 1273–1281. [Google Scholar] [CrossRef]
- Hutchings, S.C.; Foster, K.D.; Bronlund, J.E.; Lentle, R.G.; Jones, J.R.; Morgenstern, M.P. Mastication of heterogeneous foods: Peanuts inside two different food matrices. Food Qual. Prefer. 2011, 22, 332–339. [Google Scholar] [CrossRef]
- Shapiro, T.A.; Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Talalay, P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts. Cancer Epidemiol. Biomark. 2001, 10, 501. [Google Scholar]
- Feunteun, S.L.; Al-Razaz, A.; Dekker, M.; George, E.; Laroche, B.; Van Aken, G. Physiologically based modeling of food digestion and intestinal microbiota: State of the art and future challenges. An INFOGEST review. Annu. Rev. Food Sci. Technol. 2021, 12, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Crison, J.R.; Amidon, G.L. Compartmental transit and dispersion model analysis of small intestinal transit flow in humans. Int. J. Pharm. 1996, 140, 111–118. [Google Scholar] [CrossRef]
- Huang, W.; Lee, S.L.; Yu, L.X. Mechanistic approaches to predicting oral drug absorption. AAPS J. 2009, 11, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.X. An integrated model for determining causes of poor oral drug absorption. Pharm. Res. 1999, 16, 1883–1887. [Google Scholar] [CrossRef] [PubMed]
- Punt, A.; Paini, A.; Boersma, M.G.; van Bladeren, P.J.; Rietjens, I.M.C.M.; Delatour, T.; Scholz, G.; Schilter, B.; Freidig, A.P. Use of physiologically based biokinetic (PBBK) modeling to study estragole bioactivation and detoxification in humans as compared with male rats. Toxicol. Sci. 2009, 110, 255–269. [Google Scholar] [CrossRef] [Green Version]
- Punt, A.; Freidig, A.P.; Delatour, T.; Scholz, G.; Boersma, M.G.; Schilter, B.; van Bladeren, P.J.; Rietjens, I.M.C.M. A physiologically based biokinetic (PBBK) model for estragole bioactivation and detoxification in rat. Toxicol. Appl. Pharmacol. 2008, 231, 248–259. [Google Scholar] [CrossRef]
- Le Feunteun, S.; Barbé, F.; Rémond, D.; Ménard, O.; Le Gouar, Y.; Dupont, D.; Laroche, B. Impact of the dairy matrix structure on milk protein digestion kinetics: Mechanistic modelling based on mini-pig in vivo data. Food Bioprocess. Technol. 2014, 7, 1099–1113. [Google Scholar] [CrossRef]
- Strathe, A.B.; Danfær, A.; Chwalibog, A. A dynamic model of digestion and absorption in pigs. Anim. Feed. Sci. Technol. 2008, 143, 328–371. [Google Scholar] [CrossRef]
- Moxon, T.E.; Gouseti, O.; Bakalis, S. In silico modelling of mass transfer & absorption in the human gut. J. Food Eng. 2016, 176, 110–120. [Google Scholar]
- Li, Y.; Zhang, T.; Li, X.; Zou, P.; Schwartz, S.J.; Sun, D. Kinetics of sulforaphane in mice after consumption of sulforaphane-enriched broccoli sprout preparation. Mol. Nutr. Food Res. 2013, 57, 2128–2136. [Google Scholar] [CrossRef] [Green Version]
- Oliviero, T.; Verkerk, R.; Vermeulen, M.; Dekker, M. In vivo formation and bioavailability of isothiocyanates from glucosinolates in broccoli as affected by processing conditions. Mol. Nutr. Food Res. 2014, 58, 1447–1456. [Google Scholar] [CrossRef]
- Vermeulen, M.; Klöpping-Ketelaars, I.W.; van den Berg, R.; Vaes, W.H. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J. Agric. Food Chem. 2008, 56, 10505–10509. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; van Rooijen, H.J.; Vaes, W.H. Analysis of isothiocyanate mercapturic acids in urine: A biomarker for cruciferous vegetable intake. J. Agric. Food Chem. 2003, 51, 3554–3559. [Google Scholar] [CrossRef] [PubMed]
- Sreebny, L.M. Saliva in health and disease: An appraisal and update. Int. Dent. J. 2000, 50, 140–161. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.A.; Urbain, J.L.; Adler, L.P.; Charkes, N.D.; Maurer, A.H.; Krevsky, B.; Knight, L.C.; Fisher, R.S.; Malmud, L.S. Biphasic nature of gastric emptying. Gut 1988, 29, 85–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahn, A.; Angulo, A.; Cabañas, F. Purification and characterization of broccoli (Brassica oleracea var. italica) Myrosinase (β-Thioglucosidase Glucohydrolase). J. Agric. Food Chem. 2014, 62, 11666–11671. [Google Scholar] [CrossRef] [PubMed]
- Lennernäs, H. Intestinal permeability and its relevance for absorption and elimination. Xenobiotica 2007, 37, 1015–1051. [Google Scholar] [CrossRef] [PubMed]
- Román, J.; Castillo, A.; Cottet, L.; Mahn, A. Kinetic and structural study of broccoli myrosinase and its interaction with different glucosinolates. Food Chem. 2018, 254, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Dawes, C. A comparison of the effects of tasting and chewing foods on the flow rate of whole saliva in man. Arch. Oral Biol. 1988, 33, 761–764. [Google Scholar] [CrossRef]
- Bornhorst, G.M.; Singh, R.P. Bolus formation and disintegration during digestion of food carbohydrates. Compr. Rev. Food Sci. Food Saf. 2012, 11, 101–118. [Google Scholar] [CrossRef]
- Van Liere, E.J.; Sleeth, C.K.; Northup, D. The Relation of the size of the meal to the emptying time of the human stomach. Am. J. Physiol.-Leg. Content 1937, 119, 480–482. [Google Scholar] [CrossRef]
- Yu, A.; Jackson, T.; Tsume, Y.; Koenigsknecht, M.; Wysocki, J.; Marciani, L.; Amidon, G.L.; Frances, A.; Baker, J.R.; Hasler, W.; et al. Mechanistic fluid transport model to estimate gastrointestinal fluid volume and its dynamic change over time. AAPS J. 2017, 19, 1682–1690. [Google Scholar] [CrossRef]
- Helander, H.F.; Fändriks, L. Surface area of the digestive tract–revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef]
- Weiss, C.; Jelkmann, W. Functions of the blood. In Human Physiology; Schmidt, R.F., Thews, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 402–438. [Google Scholar]
- Louis, L.; Harvey, W. Predicting oral drug absorption: Mini review on physiologically-based pharmacokinetic models. Pharmaceutics 2017, 9, 41. [Google Scholar]
- Oliviero, T.; Lamers, S.; Capuano, E.; Dekker, M.; Verkerk, R. Bioavailability of isothiocyanates from broccoli sprouts in protein, lipid, and fiber gels. Mol. Nutr. Food Res. 2018, 62, 1447–1456. [Google Scholar] [CrossRef] [Green Version]
- Conaway, C.C.; Getahun, S.M.; Liebes, L.L.; Pusateri, D.J.; Topham, D.K.; Botero-Omary, M.; Chung, F.L. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr. Cancer 2000, 38, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Amidon, G.L. A compartmental absorption and transit model for estimating oral drug absorption. Int. J. Pharm. 1999, 186, 119–125. [Google Scholar] [CrossRef]
- Charron, C.S.; Vinyard, B.T.; Ross, S.A.; Seifried, H.E.; Jeffery, E.H.; Novotny, J.A. Absorption and metabolism of isothiocyanates formed from broccoli glucosinolates: Effects of BMI and daily consumption in a randomised clinical trial. Br. J. Nutr. 2018, 120, 1370–1379. [Google Scholar] [CrossRef] [Green Version]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haro, C.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Gómez-Delgado, F.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortés, J.A.; Tena-Sempere, M.; et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, e0154090. [Google Scholar] [CrossRef] [Green Version]
- Tani, N.; Ohtsuru, M.; Hata, T. Isolation of myrosinase producing microorganism. Agric. Biol. Chem. 1974, 38, 1617–1622. [Google Scholar] [CrossRef]
- Luang-In, V.; Narbad, A.; Cebeci, F.; Bennett, M.; Rossiter, J.T. Identification of proteins possibly involved in glucosinolate metabolism in L. agilis R16 and E. coli VL8. Protein J. 2015, 34, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.S.; Sirk, S.J.; Diaz, C.A.C.; Klein, A.P.; Fischer, C.R.; Higginbottom, S.K.; Erez, A.; Donia, M.S.; Sonnenburg, J.L.; Sattely, E.S. A Metabolic pathway for activation of dietary glucosinolates by a human gut symbiont. Cell 2020, 180, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Bornhorst, G.; Singh, R.; Heldman, D. Rate kinetics of bread bolus disintegration during in vitro digestion. In International Congress of Engineering and Food; Elsevier Procedia: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Kong, F.; Singh, R.P. A model stomach system to investigate disintegration kinetics of solid foods during gastric digestion. J. Food Sci. 2008, 73, 202–210. [Google Scholar] [CrossRef]
- Basile, M.; Neri, M.; Carriero, A.; Casciardi, S.; Comani, S.; Del Gratta, C.; Di Donato, L.; Di Luzio, S.; Macri, M.A.; Pasquarelli, A.; et al. Measurement of segmental transit through the gut in man. A novel approach by the biomagnetic method. Dig. Dis. Sci. 1992, 37, 1537–1543. [Google Scholar] [CrossRef]
- Degen, L.P.; Phillips, S.F. Variability of gastrointestinal transit in healthy women and men. Gut 1996, 39, 299. [Google Scholar] [CrossRef] [Green Version]





| kSH | SRR | kf | ke | ktSI | ktLI | keni | Cgl0 | ITC0 | MYR | ka | St | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HighBP | ✓ | ✓ | x | o | ✓ | x | x | o | o | o | x | x |
| HighBF | ✓ | ✓ | x | o | ✓ | x | x | o | o | o | x | x |
| MedBF | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | o | o | o | x | x |
| LowBF | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | o | o | o | x | x |
| NoBF | ✓ | x | ✓ | x | ✓ | ✓ | ✓ | o | o | o | x | x |
| Average SR Bioavailability (%) | |||
|---|---|---|---|
| Experimental Data | Model | Difference | |
| HighBP | 63 ± 0.2 | 65 ± 0.1 | 2% |
| HighBF | 33 ± 0.1 | 33 ± 0.1 | 0.1% |
| MedBF | 25 ± 0.1 | 24 ± 0.1 | 1% |
| LowBF | 19 ± 0.1 | 18 ± 0.1 | 0.9% |
| NoBF | 10 ± 0.04 | 10 ± 0.04 | 0.1% |
| High BP (n = 15) | High BF (n = 15) | Med BF (n = 14) | Low BF (n = 14) | No BF |
|---|---|---|---|---|
 |  |  |  | ---- |
| Median: 0.357 IQR: 0.251 IQR/Med: 0.7 Outliers: None | Median: 0.165 IQR: 0.223 IQR/Med: 1.4 Outliers: None | Median: 0.186 IQR: 0.13 IQR/Med: 0.7 Outliers: None | Median: 0.160 IQR: 0.143 IQR/Med: 0.9 Outliers: None | SRR parameter not fitted. Fixed at 0.047. |
| High BP(n = 15) | High BF(n = 15) | Med BF(n = 14) * | Low BF(n = 14) * | No BF(n = 14) * |
|---|---|---|---|---|
| ---- | ---- |  |  |  |
| Not fitted. kf fixed at 0.0033 min−1. | Not fitted. kf fixed at 0.0033 min−1. | Median: 0.042 min−1 IQR: 0.097 min−1 IQR/Med: 2.3 Outliers: c (0.555 min−1) | Median: 0.012 min−1 IQR: 0.018 min−1 IQR/Med: 1.5 Outliers: n (0.054 min−1) | Median: 0.003 min−1 IQR: 0.001 min−1 IQR/Med: 0.3 Outliers: g (0.006 min−1) h (0.005 min−1) q (0.021 min−1) |
| High BP(n = 15) | High BF(n = 15) | Med BF(n = 14) * | Low BF(n = 14) | No BF(n = 14) |
|---|---|---|---|---|
| ---- | ---- |  |  |  |
| Not fitted. keni fixed at 0.0015 min−1. | Not fitted. keni fixed at 0.0015 min−1. | Median: 0.017 min−1 IQR: 0.064 min−1 IQR/Med: 3.8 Outliers: c (1.95 min−1) h (0.576 min−1) n (0.26995 min−1) | Median: 0.033 min−1 IQR: 0.085 min−1 IQR/Med: 2.6 Outliers: None | Median: 0.026 min−1 IQR: 0.018 min−1 IQR/Med: 0.7 Outliers: g (0.100 min−1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shekarri, Q.; Dekker, M. A Physiological-Based Model for Simulating the Bioavailability and Kinetics of Sulforaphane from Broccoli Products. Foods 2021, 10, 2761. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10112761
Shekarri Q, Dekker M. A Physiological-Based Model for Simulating the Bioavailability and Kinetics of Sulforaphane from Broccoli Products. Foods. 2021; 10(11):2761. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10112761
Chicago/Turabian StyleShekarri, Quchat, and Matthijs Dekker. 2021. "A Physiological-Based Model for Simulating the Bioavailability and Kinetics of Sulforaphane from Broccoli Products" Foods 10, no. 11: 2761. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10112761