Preparation and Characterization of Chitosan Films Containing Lychee (Litchi chinensis Sonn.) Pericarp Powder and Their Application as Active Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
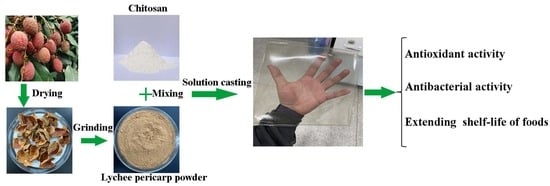
2.2. Preparation and Characterization of LPP
2.3. Film Preparation
2.4. Characterization of Films
2.4.1. Structural Characterization of the Films
2.4.2. Optical Properties of Films
2.4.3. Thickness, Moisture Content (MC), Water Solubility (WS), and Swelling Degree (SD)
2.4.4. Water Vapor Permeability (WVP)
2.4.5. Mechanical Properties
2.5. Antimicrobial Properties
2.6. Antioxidant Properties
2.7. Application as Fresh-Cut Apple Packaging
2.7.1. Weight Loss and Firmness
2.7.2. Total Soluble Solids and Titratable Acidity
2.8. Application for Preserving the Apple Juice
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of LPP
3.2. Characterization of Films
3.2.1. Structural Characterization of the Films
3.2.2. Optical Properties of Films
3.2.3. Thickness, MC, WS, and SD
3.2.4. WVP
3.2.5. Mechanical Properties
3.3. Antimicrobial Properties
3.4. Antioxidant Properties
3.5. Application as Fresh-Cut Apple Packaging
3.5.1. Physical Appearance
3.5.2. Weight Loss
3.5.3. Firmness
3.5.4. Total Soluble Solids
3.5.5. Titratable Acidity
3.6. Application for Preserving the Apple Juice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanani, Z.A.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Almasi, H.; Oskouie, M.J.; Saleh, A. A review on techniques utilized for design of controlled release food active packaging. Crit. Rev. Food Sci. Nutr. 2020, 21, 2601–2621. [Google Scholar] [CrossRef] [PubMed]
- Bastarrachea, L.J.; Wong, D.E.; Roman, M.J.; Lin, Z.S.; Goddard, J.M. Active Packaging Coatings. Coatings 2015, 5, 771–791. [Google Scholar] [CrossRef]
- Hanani, Z.A.N.; Husna, A.B.A.; Syahida, S.N.; Khaizura, M.; Jamilah, B. Effect of different fruit peels on the functional properties of gelatin/polyethylene bilayer films for active packaging. Food Packag. Shelf Life 2018, 18, 201–211. [Google Scholar] [CrossRef]
- Tian, F.; Decker, E.A.; Goddard, J.M. Controlling lipid oxidation of food by active packaging technologies. Food Funct. 2013, 4, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Z.; Ma, S.B.; Wang, Q.K.; McClements, D.J.; Liu, X.B.; Ngai, T.; Liu, F.G. Fortification of edible films with bioactive agents: A review of their formation, properties, and application in food preservation. Crit. Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef]
- Zabihzadeh Khajavi, M.; Mohammadi, R.; Ahmadi, S.; Farhoodi, M.; Yousefi, M. Strategies for controlling release of plastic compounds into foodstuffs based on application of nanoparticles and its potential health issues. Trends Food Sci. Technol. 2019, 90, 1–12. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Ozogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Alves, V.L.C.D.; Rico, B.P.M.; Cruz, R.M.S.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Preparation and characterization of a chitosan film with grape seed extract-carvacrol microcapsules and its effect on the shelf-life of refrigerated Salmon (Salmo salar). LWT 2018, 89, 525–534. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Yong, H.; Qin, Y.; Liu, J.; Jin, C. Development of antioxidant and antimicrobial packaging films based on chitosan and mangosteen (Garcinia mangostana L.) rind powder. Int. J. Biol. Macromol. 2020, 145, 1129–1139. [Google Scholar] [CrossRef]
- Pareek, S. Chapter 17—Nutritional and Biochemical Composition of Lychee (Litchi chinensis Sonn.) Cultivars. In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 395–418. [Google Scholar]
- Luo, M.; Zhang, R.; Liu, L.; Chi, J.; Huang, F.; Dong, L.; Ma, Q.; Jia, X.; Zhang, M. Preparation, stability and antioxidant capacity of nano liposomes loaded with procyandins from lychee pericarp. J. Food Eng. 2020, 284, 110065. [Google Scholar] [CrossRef]
- Ni, H.; Li, Y.H.; Hao, R.L.; Li, H.; Hu, S.Q.; Li, H.H. Identification of adenosine deaminase inhibitors from Tofu wastewater and litchi peel and their synergistic anticancer and antibacterial activities with cordycepin. Int. J. Food Sci. Technol. 2016, 51, 1168–1176. [Google Scholar] [CrossRef]
- Liu, L.; Xie, B.J.; Cao, S.Q.; Yang, E.N.; Xu, X.; Guo, S.S. A-type procyanidins from Litchi chinensis pericarp with antioxidant activity. Food Chem. 2007, 105, 1446–1451. [Google Scholar] [CrossRef]
- Maisuria, V.B.; Lopez-de Los Santos, Y.; Tufenkji, N.; Déziel, E. Cranberry-derived proanthocyanidins impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 30169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohnuma, T.; Anzai, E.; Suzuki, Y.; Shimoda, M.; Saito, S.; Nishiyama, T.; Ogura, K.; Hiratsuka, A. Selective antagonization of activated Nrf2 and inhibition of cancer cell proliferation by procyanidins from Cinnamomi Cortex extract. Arch. Biochem. Biophys. 2015, 585, 17–24. [Google Scholar] [CrossRef]
- Xu, J.; Rong, S.; Xie, B.; Sun, Z.; Zhang, L.; Wu, H.; Yao, P.; Hao, L.; Liu, L. Procyanidins Extracted from the Lotus Seedpod Ameliorate Age-Related Antioxidant Deficit in Aged Rats. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 236–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourith, N.; Kanlayavattanakul, M. Formulation and clinical evaluation of the standardized Litchi chinensis extract for skin hyperpigmentation and aging treatments. Ann. Pharm. Fr. 2020, 78, 142–149. [Google Scholar] [CrossRef]
- Liu, Z.; Du, M.; Liu, H.; Zhang, K.; Xu, X.; Liu, K.; Tu, J.; Liu, Q. Chitosan films incorporating litchi peel extract and titanium dioxide nanoparticles and their application as coatings on watercored apples. Prog. Org. Coat. 2021, 151, 106103. [Google Scholar] [CrossRef]
- Moro, T.M.A.; Ascheri, J.L.R.; Ortiz, J.A.R.; Carvalho, C.W.P.; Meléndez-Arévalo, A. Bioplastics of Native Starches Reinforced with Passion Fruit Peel. Food Bioprocess Technol. 2017, 10, 1798–1808. [Google Scholar] [CrossRef]
- De Moraes Crizel, T.; de Oliveira Rios, A.D.; Alves, V.; Bandarra, N.; Moldão-Martins, M.; Hickmann Flôres, S. Biodegradable Films Based on Gelatin and Papaya Peel Microparticles with Antioxidant Properties. Food Bioprocess Technol. 2018, 11, 536–550. [Google Scholar] [CrossRef]
- Aparicio-Fernández, X.; Vega-Ahuatzin, A.; Ochoa-Velasco, C.E.; Cid-Pérez, S.; Hernández-Carranza, P.; Ávila-Sosa, R. Physical and Antioxidant Characterization of Edible Films Added with Red Prickly Pear (Opuntia ficus-indica L.) cv. San Martín Peel and/or Its Aqueous Extracts. Food Bioprocess Technol. 2018, 11, 368–379. [Google Scholar] [CrossRef]
- Rojas-Bravo, M.; Rojas-Zenteno, E.G.; Hernández-Carranza, P.; Ávila-Sosa, R.; Aguilar-Sánchez, R.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. A Potential Application of Mango (Mangifera indica L. cv Manila) Peel Powder to Increase the Total Phenolic Compounds and Antioxidant Capacity of Edible Films and Coatings. Food Bioprocess Technol. 2019, 12, 1584–1592. [Google Scholar] [CrossRef]
- Gul, K.; Mir, N.A.; Singh, P.; Wani, A.A. Postharvest Biology and Technology of Apple. In Postharvest Biology and Technology of Temperate Fruits; Mir, S.A., Shah, M.A., Mir, M.M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 223–243. [Google Scholar]
- Rago, D.; Mette, K.; Gürdeniz, G.; Marini, F.; Poulsen, M.; Dragsted, L.O. A LC–MS metabolomics approach to investigate the effect of raw apple intake in the rat plasma metabolome. Metabolomics 2013, 9, 1202–1215. [Google Scholar] [CrossRef]
- Wu, J.L.; Sun, X.Y.; Guo, X.B.; Ji, M.Y.; Wang, J.H.; Cheng, C.; Chen, L.; Wen, C.L.; Zhang, Q.Q. Physicochemical, Antioxidant, In Vitro Release, and Heat Sealing Properties of Fish Gelatin Films Incorporated with beta-Cyclodextrin/Curcumin Complexes for Apple Juice Preservation. Food Bioprocess Technol. 2018, 11, 447–461. [Google Scholar] [CrossRef]
- Liu, J.; Jia, L.; Kan, J.; Jin, C.-H. In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem. Toxicol. 2013, 51, 310–316. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Men, H.; Tong, J.; Zhou, J. Preparation and characterization of active films based on chitosan incorporated tea polyphenols. Food Hydrocoll. 2013, 32, 35–41. [Google Scholar] [CrossRef]
- Jiang, L.; Jia, F.; Han, Y.; Meng, X.; Xiao, Y.; Bai, S. Development and characterization of zein edible films incorporated with catechin/β-cyclodextrin inclusion complex nanoparticles. Carbohydr. Polym. 2021, 261, 117877. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sui, J.; Yu, B.; Yuan, C.; Guo, L.; Abd El-Aty, A.M.; Cui, B. Physicochemical properties and antibacterial activity of corn starch-based films incorporated with Zanthoxylum bungeanum essential oil. Carbohydr. Polym. 2021, 254, 117314. [Google Scholar] [CrossRef]
- Gennadios, A.; Weller, C.L.; Gooding, C.H. Measurement errors in water vapor permeability of highly permeable, hydrophilic edible films. J. Food Eng. 1994, 21, 395–409. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, C.; Liu, X.; Zhang, N.; Xu, T.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Improvement of storage quality of strawberries by pullulan coatings incorporated with cinnamon essential oil nanoemulsion. LWT 2020, 122, 109054. [Google Scholar] [CrossRef]
- Zhang, N.; Bi, F.; Xu, F.; Yong, H.; Bao, Y.; Jin, C.; Liu, J. Structure and functional properties of active packaging films prepared by incorporating different flavonols into chitosan based matrix. Int. J. Biol. Macromol. 2020, 165, 625–634. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Jiang, W. Development of antioxidant chitosan film with banana peels extract and its application as coating in maintaining the storage quality of apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.R.; Wu, Q.; Simon, J.E. Recent Advances in Anthocyanin Analysis and Characterization. Curr. Anal. Chem. 2008, 4, 75–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liu, S.; Chen, Y.; Zhang, L.; Kan, J.; Jin, C. Physical, mechanical and antioxidant properties of chitosan films grafted with different hydroxybenzoic acids. Food Hydrocoll. 2017, 71, 176–186. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- Yang, W.; He, X.; Luzi, F.; Dong, W.; Zheng, T.; Kenny, J.M.; Puglia, D.; Ma, P. Thermomechanical, antioxidant and moisture behaviour of PVA films in presence of citric acid esterified cellulose nanocrystals. Int. J. Biol. Macromol. 2020, 161, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-I.; Zhao, Y. Development and Characterization of Edible Films from Cranberry Pomace Extracts. J. Food Sci. 2006, 71, E95–E101. [Google Scholar] [CrossRef]
- Rivero, S.; Damonte, L.; García, M.A.; Pinotti, A. An Insight into the Role of Glycerol in Chitosan Films. Food Biophys. 2016, 11, 117–127. [Google Scholar] [CrossRef]
- Zarandona, I.; Barba, C.; Guerrero, P.; de la Caba, K.; Maté, J. Development of chitosan films containing β-cyclodextrin inclusion complex for controlled release of bioactives. Food Hydrocoll. 2020, 104, 105720. [Google Scholar] [CrossRef]
- Qin, Y.-Y.; Yang, J.-Y.; Lu, H.-B.; Wang, S.-S.; Yang, J.; Yang, X.-C.; Chai, M.; Li, L.; Cao, J.-X. Effect of chitosan film incorporated with tea polyphenol on quality and shelf life of pork meat patties. Int. J. Biol. Macromol. 2013, 61, 312–316. [Google Scholar] [CrossRef]
- Hamed, S.F.; Sadek, Z.; Edris, A. Antioxidant and antimicrobial activities of clove bud essential oil and eugenol nanoparticles in alcohol-free microemulsion. J. Oleo Sci. 2012, 61, 641–648. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Erim, F.B. Total phenolic contents, antioxidant activities, and bioactive ingredients of juices from pomegranate cultivars worldwide. Food Chem. 2017, 221, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, A.; Yang, M.; Ge, Y.; Pristijono, P.; Li, J.; Xu, B.; Mi, H. Characterization of sodium alginate-based films incorporated with thymol for fresh-cut apple packaging. Food Control 2021, 126, 108063. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Z.; Shi, J.; Zou, X.; Zhai, X.; Huang, X.; Li, Z.; Holmes, M.; Daglia, M.; Xiao, J. Physical properties and bioactivities of chitosan/gelatin-based films loaded with tannic acid and its application on the preservation of fresh-cut apples. LWT 2021, 144, 111223. [Google Scholar] [CrossRef]
- Gacche, R.N.; Warangkar, S.C.; Ghole, V.S. Glutathione and Cinnamic Acid: Natural Dietary Components Used in Preventing the Process of Browning by Inhibition of Polyphenol Oxidase in Apple Juice. J. Enzym. Inhib. Med. Chem. 2004, 19, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Gull, A.; Bhat, N.; Wani, S.M.; Masoodi, F.A.; Ganai, S.A. Shelf life extension of apricot fruit by application of nanochitosan emulsion coatings containing pomegranate peel extract. Food Chem. 2021, 349, 129149. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, Y.; Wang, D.; Zhang, L.; Sun, J.; Sun, L.; Zhang, B. Effect of alginate coating combined with yeast antagonist on strawberry (Fragaria × ananassa) preservation quality. Postharvest Biol. Technol. 2009, 53, 84–90. [Google Scholar] [CrossRef]
- Yang, G.; Yue, J.; Gong, X.; Qian, B.; Wang, H.; Deng, Y.; Zhao, Y. Blueberry leaf extracts incorporated chitosan coatings for preserving postharvest quality of fresh blueberries. Postharvest Biol. Technol. 2014, 92, 46–53. [Google Scholar] [CrossRef]









| Films | L | a | b | ΔE |
|---|---|---|---|---|
| CHS film | 87.47 ± 1.18 a | −2.49 ± 0.09 e | 0.13 ± 0.12 e | 0.00 ± 0.00 e |
| CHS-2.5LPP | 81.77 ± 0.55 b | −1.47 ± 0.15 d | 6.87 ± 0.81 d | 8.91 ± 0.51 d |
| CHS-5LPP | 79.31 ± 0.19 c | −0.17 ± 0.12 c | 11.83 ± 1.52 c | 14.48 ± 1.03 c |
| CHS-7.5LPP | 76.41 ± 0.56 d | 1.67 ± 0.23 b | 20.59 ± 0.62 b | 23.65 ± 0.29 b |
| CHS-10LPP | 74.09 ± 0.66 e | 2.91 ± 0.09 a | 25.17 ± 1.34 a | 28.91 ± 0.77 a |
| Films | Thickness (mm) | MC (%) | WS (%) | SD (%) |
|---|---|---|---|---|
| CHS film | 0.058 ± 0.006 e | 31.663 ± 0.272 a | 20.487 ± 0.798 a | 164.547 ± 3.868 a |
| CHS-2.5LPP | 0.071 ± 0.007 d | 26.041 ± 0.869 b | 18.341 ± 0.251 b | 128.501 ± 2.265 b |
| CHS-5LPP | 0.082 ± 0.003 c | 17.107 ± 0.816 c | 16.263 ± 0.411 c | 100.687 ± 4.642 c |
| CHS-7.5LPP | 0.097 ± 0.009 b | 16.583 ± 0.112 c | 15.223 ± 0.172 d | 73.433 ± 3.365 d |
| CHS-10LPP | 0.115 ± 0.003 a | 16.581 ± 0.434 c | 14.342 ± 0.251 e | 56.971 ± 1.758 e |
| Films | WVP (×10−7 g m−1 h−1 Pa−1) | TS (MPa) | EAB (%) | EM (MPa) |
|---|---|---|---|---|
| CHS film | 6.67 ± 0.09 a | 10.98 ± 0.56 e | 24.79 ± 1.59 a | 44.41 ± 2.61 e |
| CHS-2.5LPP | 6.44 ± 0.07 b | 12.47 ± 0.11 d | 19.85 ± 0.62 b | 62.86 ± 1.83 d |
| CHS-5LPP | 6.13 ± 0.11 c | 14.28 ± 0.09 c | 18.35 ± 0.45 c | 77.84 ± 1.14 c |
| CHS-7.5LPP | 5.41 ± 0.09 d | 16.06 ± 0.16 b | 14.51 ± 0.42 d | 110.74 ± 2.46 b |
| CHS-10LPP | 4.67 ± 0.06 e | 16.82 ± 0.39 a | 9.58 ± 0.63 e | 176.25 ± 12.61 a |
| Films | Diameter of Inhibition Zone (mm) | |||
|---|---|---|---|---|
| Escherichia coli | Staphylococcus aureus | DPPH Radical Scavenging Activity (%) | Total Antioxidant Activity (%) | |
| CHS film | 10.81 ± 0.16 e | 11.03 ± 0.12 e | - | - |
| CHS-2.5LPP | 11.81 ± 0.06 d | 12.04 ± 0.08 d | 45.07 ± 1.37 d | 54.52 ± 1.24 d |
| CHS-5LPP | 12.45 ± 0.08 c | 12.72 ± 0.09 c | 55.53 ± 0.99 c | 64.47 ± 1.55 c |
| CHS-7.5LPP | 13.18 ± 0.11 b | 13.48 ± 0.08 b | 65.06 ± 1.36 b | 74.41 ± 1.14 b |
| CHS-10LPP | 14.02 ± 0.08 a | 14.41 ± 0.09 a | 70.09 ± 1.37 a | 82.29 ± 1.54 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Luo, Z.; Liu, H.; Wang, F.; Li, H.; Gao, H.; Zhang, H. Preparation and Characterization of Chitosan Films Containing Lychee (Litchi chinensis Sonn.) Pericarp Powder and Their Application as Active Food Packaging. Foods 2021, 10, 2834. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10112834
Jiang L, Luo Z, Liu H, Wang F, Li H, Gao H, Zhang H. Preparation and Characterization of Chitosan Films Containing Lychee (Litchi chinensis Sonn.) Pericarp Powder and Their Application as Active Food Packaging. Foods. 2021; 10(11):2834. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10112834
Chicago/Turabian StyleJiang, Longwei, Zhao Luo, Haibi Liu, Fenghui Wang, Hanyu Li, Hechen Gao, and Huajiang Zhang. 2021. "Preparation and Characterization of Chitosan Films Containing Lychee (Litchi chinensis Sonn.) Pericarp Powder and Their Application as Active Food Packaging" Foods 10, no. 11: 2834. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10112834