Alternatives to Cow’s Milk-Based Infant Formulas in the Prevention and Management of Cow’s Milk Allergy
Abstract
:1. Introduction
2. Food Allergy
2.1. Cow’s Milk Allergy
2.2. Prevention and Management of Cow’s Milk Allergy
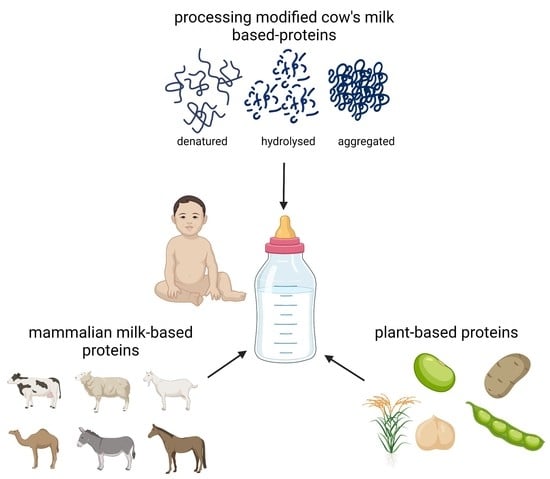
3. Infant Formulas
4. Cow’s Milk-Based Infant Formulas
4.1. Reduction of Cow’s Milk Protein Allergenicity by Process Modifications
4.1.1. Enzymatic Hydrolysis
4.1.2. Fermentation
4.1.3. Heat Treatment
4.1.4. High Pressure
4.1.5. Radiation
4.1.6. Other Processing Technologies
5. Amino Acid-Based Infant Formulas
6. Infant Formulas Based on Mammalian Milk Proteins
6.1. Goat Milk
6.2. Sheep Milk
6.3. Camel Milk
6.4. Donkey Milk
6.5. Horse Milk
7. Plant-Based Infant Formulas
7.1. Soy-Based Infant Formulas
7.2. Hydrolysed Rice-Based Infant Formulas
7.3. Potential Future Plant-Based Infant Formulas
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiocchi, A.; Dahda, L.; Dupont, C.; Campoy, C.; Fierro, V.; Nieto, A. Cow’s milk allergy: Towards an update of DRACMA guidelines. World Allergy Organ. J. 2016, 9, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arenz, S.; Rückerl, R.; Koletzko, B.; Von Kries, R. Breast-feeding and childhood obesity—A systematic review. Int. J. Obes. 2004, 28, 1247–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belfort, M.B. The Science of Breastfeeding and Brain Development. Breastfeed. Med. 2017, 12, 459–461. [Google Scholar] [CrossRef] [PubMed]
- WHO. Infant and young child nutrition: Global strategy on infant and young child feeding. In Proceedings of the Fifty Fifth World Health Assembly, Geneva, Switzerland, 13–18 May 2002; Volume 53, pp. 1–18. [Google Scholar]
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of infant feeding: Key features of breast milk and infant formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Comission. Commission Directive 2006/141/EC of 22 December on infant formulae and follow-on formulae and amending Directive 1999/21/EC. Off. J. Eur. Union 2006, 49, 1–33. [Google Scholar]
- European Comission. Commission Directive 2013/46/EU of 28 August 2013 amending Directive 2006/141/EC with regard to protein requirements for infant formulae and follow-on formulae. Off. J. Eur. Union 2013, 46, 16–19. [Google Scholar]
- Nutten, S.; Maynard, F.; Järvi, A.; Rytz, A.; Simons, P.J.; Heine, R.G.; Kuslys, M. Peptide size profile and residual immunogenic milk protein or peptide content in extensively hydrolyzed infant formulas. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 1446–1449. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, K.S.; Parker, M.; Ameerally, A.; Drake, S.L.; Drake, M.A. Drivers of choice for fluid milk versus plant-based alternatives: What are consumer perceptions of fluid milk? J. Dairy Sci. Off. Publ. Am. Dairy Sci. Assoc. 2017, 100, 6125–6138. [Google Scholar] [CrossRef]
- Silva, A.R.A.; Silva, M.M.N.; Ribeiro, B.D. Health issues and technological aspects of plant-based alternative milk. Food Res. Int. 2020, 131, 108972. [Google Scholar] [CrossRef]
- Dahdah, L.; Sato, S.; Hossny, E.; Irani, C.; Said, M.; Scadding, G. The Global Problem of food allergy—Information Sheet. World Allergy WeekWorld Allergy Week; pp. 1–8. 2019. Available online: https://www.worldallergy.org/UserFiles/file/WAO-2019-Food-Allergy-Information-Sheet.pdf (accessed on 15 January 2022).
- Tang, M.L.K.; Mullins, R.J. Food allergy: Is prevalence increasing? Intern. Med. J. 2016, 47, 256–261. [Google Scholar] [CrossRef]
- INFOSAN IFSAN. Information Note No. 3/2006—Food Allergies; pp. 9–12. 2006. Available online: https://www.who.int/foodsafety/fs_management/No_03_allergy_June06_en.pdf (accessed on 15 January 2022).
- Teuber, S.; Beyer, K.; Comstock, S.; Wallowitz, M. The Big Eight Foods: Clinical and Epidemiological Overview. In Food Allergy; ASM Press: Washington, DC, USA, 2006; pp. 49–79. [Google Scholar]
- Zepeda-Ortega, B.; Goh, A.; Xepapadaki, P.; Sprikkelman, A.; Nicolaou, N.; Hernandez, R.E.H.; Latiff, A.H.A.; Yat, M.T.; Diab, M.; Hussaini, B.A. Strategies and Future Opportunities for the Prevention, Diagnosis, and Management of Cow Milk Allergy. Front. Immunol. 2021, 12, 1877. [Google Scholar] [CrossRef] [PubMed]
- Loh, W.; Tang, M.L.K. The epidemiology of food allergy in the global context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, G.; Berti, I.; Burks, A.W.; Krauss, B.; Barbi, E. IgE-mediated food allergy in children. Lancet 2013, 382, 1656–1664. [Google Scholar] [CrossRef]
- Skripak, J.M.; Matsui, E.C.; Mudd, K.; Wood, R.A. The natural history of IgE-mediated cow’s milk allergy. J. Allergy Clin. Immunol. 2007, 120, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Ponce, M.; Diesner, S.C.; Szépfalusi, Z.; Eiwegger, T. Markers of tolerance development to food allergens. Allergy Eur. J. Allergy Clin. Immunol. 2016, 71, 1393–1404. [Google Scholar] [CrossRef] [Green Version]
- Berin, M.C. Mechanisms that define transient versus persistent food allergy. J. Allergy Clin. Immunol. 2019, 143, 453–457. [Google Scholar] [CrossRef]
- Sampson, H.A.; O’Mahony, L.; Burks, A.W.; Plaut, M.; Lack, G.; Akdis, C.A. Mechanisms of food allergy. J. Allergy Clin. Immunol. 2018, 141, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.S.; Lau, C.H.; Sita, E.E.; Smith, B.; Greenhawt, M.J. Factors associated with reported food allergy tolerance among US children. Ann. Allergy Asthma Immunol. 2013, 111, 194–198.e4. [Google Scholar] [CrossRef]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; Dutoit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and management of food allergy. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 1008–1025. [Google Scholar] [CrossRef]
- Anagnostou, K. Oral Immunotherapy for Food Allergy: What Have We Achieved So Far? EMJ Allergy Immunol. 2017, 2, 94–99. [Google Scholar]
- Burbank, A.J.; Sood, P.; Vickery, B.P.; Wood, R.A. Oral Immunotherapy for Food Allergy. Immunol. Allergy Clin. N. Am. 2016, 36, 55–69. [Google Scholar] [CrossRef] [PubMed]
- ADP101 for Oral Immunotherapy in Food-Allergic Children and Adults. Available online: https://clinicaltrials.gov/ct2/show/results/NCT04856865 (accessed on 15 January 2022).
- Food and Drug Administration. PALFORZIA. Available online: https://www.fda.gov/vaccines-blood-biologics/allergenics/palforzia (accessed on 15 January 2022).
- Vierk, K.A.; Koehler, K.M.; Fein, S.B.; Street, D.A. Prevalence of self-reported food allergy in American adults and use of food labels. J. Allergy Clin. Immunol. 2007, 119, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 2014, 133, 291–307.e5. [Google Scholar] [CrossRef]
- Kazatsky, A.M.; Wood, R.A. Classification of Food Allergens and Cross-Reactivity. Curr. Allergy Asthma Rep. 2016, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.L.; Walkner, M.; Vickery, B.P.; Gupta, R.S. Clinical Management of Food Allergy. Pediatr. Clin. N. Am. 2015, 62, 1409–1424. [Google Scholar] [CrossRef] [Green Version]
- Lieberman, J.A.; Sicherer, S.H. Quality of life in food allergy. Curr. Opin. Allergy Clin. Immunol. 2011, 11, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A.; Weiss, C.; Furlong, T.J.; Sicherer, M.; Sicherer, S.H. Bullying among pediatric patients with food allergy. Ann. Allergy Asthma Immunol. 2010, 105, 282–286. [Google Scholar] [CrossRef]
- Rajan, J.; Laubach, S. Child and Parental Reports of Bullying in a Consecutive Sample of Children With Food Allergy. Pediatrics 2013, 132, 23–24. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef]
- Czolk, R.; Klueber, J.; Sørensen, M.; Wilmes, P.; Codreanu-Morel, F.; Skov, P.S.; Hilger, C.; Bindslev-Jensen, C.; Ollert, M.; Kuehn, A. IgE-Mediated Peanut Allergy: Current and Novel Predictive Biomarkers for Clinical Phenotypes Using Multi-Omics Approaches. Front. Immunol. 2021, 11, 3653. [Google Scholar] [CrossRef]
- Broekman, H.C.H.; Eiwegger, T.; Upton, J.; Bøgh, K.L. IgE—The main player of food allergy. Drug Discov. Today Dis. Model 2015, 17–18, 37–44. [Google Scholar] [CrossRef]
- Divekar, R.; Kita, H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 98–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stark, J.M.; Tibbitt, C.A.; Coquet, J.M. The metabolic requirements of Th2 cell differentiation. Front. Immunol. 2019, 10, 2318. [Google Scholar] [CrossRef] [PubMed]
- Humeniuk, P.; Dubiela, P.; Hoffmann-Sommergruber, K. Dendritic cells and their role in allergy: Uptake, proteolytic processing and presentation of allergens. Int. J. Mol. Sci. 2017, 18, 71491. [Google Scholar] [CrossRef] [Green Version]
- Anvari, S.; Miller, J.; Yeh, C.Y.; Davis, C.M. IgE-Mediated Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 244–260. [Google Scholar] [CrossRef] [Green Version]
- Satitsuksanoa, P.; Daanje, M.; Akdis, M.; Boyd, S.D.; van de Veen, W. Biology and dynamics of B cells in the context of IgE-mediated food allergy. Allergy Eur. J. Allergy Clin. Immunol. 2021, 76, 1707–1717. [Google Scholar] [CrossRef]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children—EuroPrevall birth cohort. Allergy Eur. J. Allergy Clin. Immunol. 2015, 70, 963–972. [Google Scholar] [CrossRef]
- Flom, J.D.; Sicherer, S.H. Epidemiology of cow’s milk allergy. Nutrients 2019, 11, 1051. [Google Scholar] [CrossRef] [Green Version]
- Høst, A. Frequency of cow’s milk allergy in childhood. Ann. Allergy Asthma Immunol. 2002, 89, 33–37. [Google Scholar] [CrossRef]
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Spitzauer, S.; Valenta, R. Cow’s milk allergy: From allergens to new forms of diagnosis, therapy and prevention. Methods 2014, 66, 22–33. [Google Scholar] [CrossRef]
- Walsh, J.; Meyer, R.; Shah, N.; Quekett, J.; Fox, A.T. Differentiating milk allergy (IgE and non-IgE mediated) from lactose intolerance: Understanding the underlying mechanisms and presentations. Br. J. Gen. Pract. 2016, 66, e609–e611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiocchi, A.; Dahdah, L.; Albarini, M.; Martelli, A. Cow’s milk allergy in children and adults. Chem. Immunol. Allergy 2015, 101, 114–123. [Google Scholar] [PubMed]
- Kalach, N.; Bellaïche, M.; Elias-Billon, I.; Dupont, C. Family history of atopy in infants with cow’s milk protein allergy: A French population-based study. Arch. Pediatr. 2019, 26, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, E.; Zhu, P.; Schuck, P. Infant formula powders. In Handbook of Food Powders: Processes and Properties; Woodhead Publishing Limited: Sawston, UK, 2013. [Google Scholar] [CrossRef]
- Malacarne, M.; Martuzzi, F.; Summer, A.; Mariani, P. Protein and fat composition of mare’s milk: Some nutritional remarks with reference to human and cow’s milk. Int. Dairy J. 2002, 12, 869–877. [Google Scholar] [CrossRef]
- Bøgh, K.L.; Larsen, J.M. Chapter 19: Reducing allergenicity by proteolysis. In Agents of Change: Enzymes in Milk and Dairy Products; Springer: Berlin/Heidleberg, Germany, 2021; pp. 499–524. Available online: http://0-link-springer-com.brum.beds.ac.uk/10.1007/978-3-030-55482-8 (accessed on 15 January 2022).
- Hurley, W.L.; Theil, P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef]
- Pomés, A.; Davies, J.M.; Gadermaier, G.; Hilger, C.; Holzhauser, T.; Lidholm, J.; Lopata, A.L.; Mueller, G.A.; Nandy, A.; Radauer, C.; et al. WHO/IUIS Allergen Nomenclature: Providing a common language. Mol. Immunol. 2018, 100, 3–13. [Google Scholar] [CrossRef]
- WHO/IUIS. Allergen Nomenclature Sub-Committee. Available online: http://allergen.org/ (accessed on 5 January 2022).
- Smyth, E.; Clegg, R.A.; Holt, C. A biological perspective on the structure and function of caseins and casein micelles. Int. J. Dairy Technol. 2004, 57, 121–126. [Google Scholar] [CrossRef]
- Holt, C.; Carver, J.A.; Ecroyd, H.; Thorn, D.C. Invited review: Caseins and the casein micelle: Their biological functions, structures, and behavior in foods1. J. Dairy Sci. 2013, 96, 6127–6146. [Google Scholar] [CrossRef]
- Holzmüller, W.; Gmach, O.; Griebel, A.; Kulozik, U. Casein precipitation by acid and rennet coagulation of buttermilk: Impact of pH and temperature on the isolation of milk fat globule membrane proteins. Int. Dairy J. 2016, 63, 115–123. [Google Scholar] [CrossRef]
- Corredig, M.; Dalgleish, D.G. The mechanisms of the heat-induced interaction of whey proteins with casein micelles in milk. Int. Dairy J. 1999, 9, 233–236. [Google Scholar] [CrossRef]
- Chapman, M. Allergen nomenclature. Clin. Allergy Immunol. 2008, 21, 47–58. [Google Scholar] [PubMed]
- Monaci, L.; Tregoat, V.; van Hengel, A.J.; Anklam, E. Milk allergens, their characteristics and their detection in food: A review. Eur. Food Res. Technol. 2006, 223, 149–179. [Google Scholar] [CrossRef]
- Vicente-Serrano, J.; Caballero, M.L.; Rodríguez-Pérez, R.; Carretero, P.; Pérez, R.; Blanco, J.G.; Juste, S.; Moneo, I. Sensitization to serum albumins in children allergic to cow’s milk and epithelia. Pediatr. Allergy Immunol. 2007, 18, 503–507. [Google Scholar] [CrossRef]
- Villa, C.; Costa, J.; Oliveira, M.B.P.P.; Mafra, I. Bovine Milk Allergens: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 137–164. [Google Scholar] [CrossRef] [Green Version]
- Negaoui, H.; El Mecherfi, K.E.; Tadjer, S.A.; Grar, H.; Kheroua, O.; Saidi, D. Bovine lactoferrin allergenicity as studied in murine model of allergy. Food Agric. Immunol. 2016, 27, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Gaudin, J.C.; Rabesona, H.; Choiset, Y.; Yeretssian, G.; Chobert, J.M.; Sakanyan, V.; Drouet, M.; Haertlé, T. Assessment of the immunoglobulin E-mediated immune response to milk-specific proteins in allergic patients using microarrays. Clin. Exp. Allergy 2008, 38, 686–693. [Google Scholar] [CrossRef]
- Natale, M.; Bisson, C.; Monti, G.; Peltran, A.; Garoffo, L.P.; Valentini, S.; Fabris, C.; Bertino, E.; Coscia, A.; Conti, A. Cow’s milk allergens identification by two-dimensional immunoblotting and mass spectrometry. Mol. Nutr. Food Res. 2004, 48, 363–369. [Google Scholar] [CrossRef]
- Van Regenmortel, M.H.V. What is a B-cell epitope? Methods Mol. Biol. 2009, 524, 3–20. [Google Scholar]
- Arnon, R.; Van Regenmortel, M.H.V. Structural basis of antigenic specificity and design of new vaccines. FASEB J. 1992, 6, 3265–3274. [Google Scholar] [CrossRef]
- Ansari, H.R.; Raghava, G.P. Identification of conformational B-cell Epitopes in an antigen from its primary sequence. Immunome Res. 2010, 6, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, E.A.; Calder, P.C. Maternal diet and its influence on the development of allergic disease. Clin. Exp. Allergy 2015, 45, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kim, K.W.; Choi, B.S.; Jee, H.M.; Sohn, M.H.; Kim, K.E. Relationship between mode of delivery in childbirth and prevalence of allergic diseases in Korean children. Allergy Asthma Immunol. Res. 2010, 2, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeever, T.M.; Lewis, S.A.; Smith, C.; Hubbard, R. Mode of delivery and risk of developing allergic disease. J. Allergy Clin. Immunol. 2002, 109, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, E.A. Probiotics and food allergy. Vopr. Prakt. Pediatr. 2016, 11, 60–64. [Google Scholar] [CrossRef]
- Fox, A.; Bird, J.A.; Fiocchi, A.; Knol, J.; Meyer, R.; Salminen, S.; Sitang, G.; Szajewska, H.; Papadopoulos, N. The potential for pre-, pro- and synbiotics in the management of infants at risk of cow’s milk allergy or with cow’s milk allergy: An exploration of the rationale, available evidence and remaining questions. World Allergy Organ. J. 2019, 12, 100034. [Google Scholar] [CrossRef] [Green Version]
- Vandenplas, Y. Prevention and management of cow’s milk allergy in non-exclusively breastfed infants. Nutrients 2017, 9, 731. [Google Scholar] [CrossRef]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Perkin, M.R.; Logan, K.; Marrs, T.; Radulovic, S.; Craven, J.; Flohr, C.; Lack, G. Enquiring about Tolerance (EAT) study: Feasibility of an early allergenic food introduction regimen. J. Allergy Clin. Immunol. 2016, 137, 1477–1486.e8. [Google Scholar] [CrossRef] [Green Version]
- Koplin, J.J.; Osborne, N.J.; Wake, M.; Martin, P.E.; Gurrin, L.C.; Robinson, M.N.; Tey, D.; Slaa, M.; Thiele, L.; Miles, L.; et al. Can early introduction of egg prevent egg allergy in infants? A population-based study. J. Allergy Clin. Immunol. 2010, 126, 807–813. [Google Scholar] [CrossRef]
- American Academy of Pediatrics C of, N. Hypoallergenic Infant Formulas. Pediatrics 2000, 106, 346–349. [Google Scholar]
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R.; et al. EAACI guideline: Preventing the development of food allergy in infants and young children (2020 update). Pediatr. Allergy Immunol. 2021, 32, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Tang, M.L.K. The Australasian Society of Clinical Immunology and Allergy position statement: Summary of allergy prevention in children. Med. J. Aust. 2005, 182, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.A.; Smith, J.; Vale, S.; Campbell, D.E. The Australasian Society of Clinical Immunology and Allergy infant feeding for allergy prevention guidelines. Med. J. Aust. 2019, 210, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, D.M.; Chan, E.S.; Venter, C.; Spergel, J.M.; Abrams, E.M.; Stukus, D.; Groetch, M.; Shaker, M.; Greenhawt, M. A Consensus Approach to the Primary Prevention of Food Allergy Through Nutrition: Guidance from the American Academy of Allergy, Asthma, and Immunology; American College of Allergy, Asthma, and Immunology; and the Canadian Society for Allergy and Clinical Immunology. J. Allergy Clin. Immunol. Pract. 2021, 9, 22–43.e4. [Google Scholar] [CrossRef]
- Kramer, M.S.; Kakuma, R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Evid.-Based Child Health 2014, 9, 447–483. [Google Scholar] [CrossRef]
- Tuokkola, J.; Luukkainen, P.; Tapanainen, H.; Kaila, M.; Vaarala, O.; Kenward, M.G.; Virta, L.J.; Veijola, R.; Simell, O.; Ilonen, J.; et al. Maternal diet during pregnancy and lactation and cow’s milk allergy in offspring. Eur. J. Clin. Nutr. 2016, 70, 554–559. [Google Scholar] [CrossRef]
- Stråvik, M.; Barman, M.; Hesselmar, B.; Sandin, A.; Wold, A.E.; Sandberg, A.S. Maternal intake of cow’s milk during lactation is associated with lower prevalence of food allergy in offspring. Nutrients 2020, 12, 3680. [Google Scholar] [CrossRef]
- Ewen, C. C-section babies are missing key microbes. Nature 2019. [Google Scholar]
- Selma-Royo, M.; Tarrazó, M.; García-Mantrana, I.; Gómez-Gallego, C.; Salminen, S.; Carmen Collado, M. Shaping Microbiota During the First 1000 Days of Life. In Probiotics and Child Gastrointestinal Health; Springer: Berlin/Heidelberg, Germany, 2019; pp. 3–24. [Google Scholar]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef] [Green Version]
- Adeyeye, T.E.; Yeung, E.H.; McLain, A.C.; Lin, S.; Lawrence, D.A.; Bell, E.M. Wheeze and Food Allergies in Children Born via Cesarean Delivery. Am. J. Epidemiol. 2019, 188, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Mitselou, N.; Hallberg, J.; Stephansson, O.; Almqvist, C.; Melén, E.; Ludvigsson, J.F. Cesarean delivery, preterm birth, and risk of food allergy: Nationwide Swedish cohort study of more than 1 million children. J. Allergy Clin. Immunol. 2018, 142, 1510–1514.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyrhönen, K.; Kulmala, P. Delivery mode and the incidence of atopic sensitization and food allergy in a Finnish child population. Pediatr. Allergy Immunol. 2021, 33, e13584. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, B.; Kirley, K.; Mounsey, A.; Ewigman, B. Prescribing an antibiotic? Pair it with probiotics. J. Fam. Pract. 2013, 62, 148–150. [Google Scholar]
- Prescott, S.L.; Wiltschut, J.; Taylor, A.; Westcott, L.; Jung, W.; Currie, H.; Dunstan, J.A. Early markers of allergic disease in a primary prevention study using probiotics: 2.5-Year follow-up phase. Allergy Eur. J. Allergy Clin. Immunol. 2008, 63, 1481–1490. [Google Scholar] [CrossRef]
- Plummer, E.L.; Chebar Lozinsky, A.; Tobin, J.M.; Uebergang, J.B.; Axelrad, C.; Garland, S.M.; Jacobs, S.E.; Tang, M.L.K. Postnatal probiotics and allergic disease in very preterm infants: Sub-study to the ProPrems randomized trial. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 127–136. [Google Scholar] [CrossRef]
- WHO. Infant and Young Child Feeding. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding#:~:text= (accessed on 2 February 2022).
- Du Toit, G.; Katz, Y.; Sasieni, P.; Mesher, D.; Maleki, S.J.; Fisher, H.R.; Fox, A.T.; Turcanu, V.; Amir, T.; Zadik-Mnuhin, G.; et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J. Allergy Clin. Immunol. 2008, 122, 984–991. [Google Scholar] [CrossRef]
- Yakaboski, E.; Robinson, L.B.; Arroyo, A.; Espinola, J.A.; Geller, R.J.; Sullivan, A.F.; Rudders, S.A.; Camargo, C.A. Early Introduction of Food Allergens and Risk of Developing Food Allergy. Nutrients 2021, 20, 216. [Google Scholar] [CrossRef]
- Abrams, E.M.; Sicherer, S.H. Cow’s milk allergy prevention. Ann. Allergy Asthma Immunol. 2021, 127, 36–41. [Google Scholar] [CrossRef]
- Katz, Y.; Rajuan, N.; Goldberg, M.R.; Eisenberg, E.; Heyman, E.; Cohen, A.; Leshno, M. Early exposure to cow’s milk protein is protective against IgE-mediated cow’s milk protein allergy. J. Allergy Clin. Immunol. 2010, 126, 77–82.e1. [Google Scholar] [CrossRef]
- Chan, E.S.; Abrams, E.M.; Hildebrand, K.J.; Watson, W. Early introduction of foods to prevent food allergy. Allergy Asthma Clin. Immunol. 2018, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Osborn, D.A.; Sinn, J.K.H.; Jones, L.J. Infant formulas containing hydrolysed protein for prevention of allergic disease. Cochrane Database Syst Rev. 2018, 2018, 1–119. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: Espghan gi committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Brozek, J.; Schu, H.; Von Berg, A.; Beyer, K.; Bozzola, M.; Bradsher, J.; Compalati, E.; Ebisawa, M.; Guzman, M.A.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines. Pediatr. Allergy Immunol. 2010, 21, 1–125. [Google Scholar] [CrossRef] [Green Version]
- Vandenplas, Y.; Brough, H.A.; Fiocchi, A.; Miqdady, M.; Munasir, Z.; Salvatore, S.; Thapar, N.; Venter, C.; Vieira, M.C.; Meyer, R. Current Guidelines and Future Strategies for the Management of Cow’s Milk Allergy. J. Asthma Allergy 2021, 14, 1243–1256. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Brueton, M.; Dupont, C.; Hill, D.; Isolauri, E.; Koletzko, S.; Oranje, A.P.; Staiano, A. Guidelines for the diagnosis and management of cow’s milk protein allergy in infants. Arch. Dis. Child. 2007, 92, 902–908. [Google Scholar] [CrossRef] [Green Version]
- Høst, A.; Husby, S.; Hansen, L.G.; Østerballe, O. Bovine β-lactoglobulin in human milk from atopic and non-atopic mothers. Relationship to maternal intake of homogenized and unhomogenized milk. Clin. Exp. Allergy 1990, 20, 383–387. [Google Scholar] [CrossRef]
- Ludman, S.; Shah, N.; Fox, A.T. Managing cows’ milk allergy in children. BMJ 2013, 347, f5424. [Google Scholar] [CrossRef] [Green Version]
- Chruszcz, M.; Mikolajczak, K.; Mank, N.; Majorek, K.A.; Porebski, P.J.; Minor, W. Serum albumins—Unusual allergens. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 5375–5381. [Google Scholar] [CrossRef] [Green Version]
- WHO. Codex Alimentarius: Standard for Infant Formula and Formulas For Special Medical Purposes Intended For Infants; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Sharif, M.K.; Zahid, A.; Shah, F.-u.-H. Role of Food Product Development in Increased Food Consumption and Value Addition. In Food Processing for Increased Quality and Consumption; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- EU Commission. Regulations. Commission delegated regulation (EU) 2016/127. Off. J. Eur. Union 2016, L25, 1–29. [Google Scholar]
- WHO/UNICEF. International Code for Marketing Breastmilk Substitutes; WHO: Geneva, Switzerland, 1981. [Google Scholar]
- Koletzko, B.; Beyer, J.; Brands, B.; Demmelmair, H.; Grote, V.; Haile, G.; Gruszfeld, D.; Rzehak, P.; Socha, P.; Weber, M. Early influences of nutrition on postnatal growth. Nestle Nutr. Inst. Workshop Ser. 2013, 71, 11–27. [Google Scholar]
- Leung, A.K.C.; Sauve, R.S. Whole cow’s milk in infancy. Paediatr. Child Health 2003, 8, 419–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, J.; Greer, F. Use of soy protein-based formulas in infant feeding. Pediatrics 2008, 121, 1062–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restani, P.; Ballabio, C.; Di Lorenzo, C.; Tripodi, S.; Fiocchi, A. Molecular aspects of milk allergens and their role in clinical events. Anal. Bioanal. Chem. 2009, 395, 47–56. [Google Scholar] [CrossRef]
- Ye, A.; Cui, J.; Carpenter, E.; Prosser, C.; Singh, H. Dynamic in vitro gastric digestion of infant formulae made with goat milk and cow milk: Influence of protein composition. Int. Dairy J. 2019, 97, 76–85. [Google Scholar] [CrossRef]
- Jia, K.; Feng, Y.; Brenna, J.T.; Luo, Z.C.; Zhao, J.; Li, H.; Li, P.; Zhang, Q.; Zhao, Q.; Dai, Q.; et al. Breast milk EPA associated with infant distractibility when EPA level is low. Nutrition 2021, 86, 111143. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, L.A.; Yeo, Y.K. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 1999, 40, 211–225. [Google Scholar] [CrossRef] [Green Version]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits—A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef]
- Bryś, J.; Górska, A.; Ostrowska-Ligęza, E.; Wirkowska-Wojdyła, M.; Bryś, A.; Brzezińska, R.; Dolatowska-żebrowska, K.; Małajowicz, J.; Ziarno, M.; Obranović, M.; et al. Human milk fat substitutes from lard and hemp seed oil mixtures. Appl. Sci. 2021, 11, 7014. [Google Scholar] [CrossRef]
- Parschat, K.; Melsaether, C.; Jäpelt, K.R.; Jennewein, S. Clinical evaluation of 16-week supplementation with 5HMO-mix in healthy-term human infants to determine tolerability, safety, and effect on growth. Nutrients 2021, 13, 2871. [Google Scholar] [CrossRef]
- Bakker-Zierikzee, A.M.; Alles, M.S.; Knol, J.; Kok, F.J.; Tolboom, J.J.M.; Bindels, J.G. Effects of infant formula containing a mixture of galacto- and fructo-oligosaccharides or viable Bifidobacterium animalis on the intestinal microflora during the first 4 months of life. Br. J. Nutr. 2005, 94, 783–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pehrsson, P.R.; Patterson, K.Y.; Khan, M.A. Selected vitamins, minerals and fatty acids in infant formulas in the United States. J. Food Compos. Anal. 2014, 36, 66–71. [Google Scholar] [CrossRef]
- Green Corkins, K.; Shurley, T. What’s in the Bottle? A Review of Infant Formulas. Nutr. Clin. Pract. 2016, 31, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, Structure, and Digestive Dynamics of Milk From Different Species—A Review. Front. Nutr. 2020, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Ahmad, S. Formulation guidelines for infant formula. In Human Milk Biochemistry and Infant Formula Manufacturing Technology; Woodhead Publishing Limited: Sawston, UK, 2014. [Google Scholar] [CrossRef]
- Happe, R.P.; Gambelli, L. Infant formula. In Specialty Oils and Fats in Food and Nutrition: Properties, Processing and Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Focke-Tejkl, M.; Reininger, R.; Civaj, V.; Campana, R.; Thalhamer, J.; Scheiblhofer, S.; Balic, N.; et al. Infant milk formulas differ regarding their allergenic activity and induction of T-cell and cytokine responses. Allergy Eur. J. Allergy Clin. Immunol. 2017, 72, 416–424. [Google Scholar] [CrossRef] [Green Version]
- Fenelon, M.A.; Hickey, R.M.; Buggy, A.; McCarthy, N.; Murphy, E.G. Whey proteins in infant formula. In Whey Proteins: From Milk to Medicine; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 439–494. [Google Scholar] [CrossRef]
- Golkar, A.; Milani, J.M.; Vasiljevic, T. Altering allergenicity of cow’s milk by food processing for applications in infant formula. Crit. Rev. Food Sci. Nutr. 2019, 59, 159–172. [Google Scholar] [CrossRef]
- Host, A.; Halken, S. Hypoallergenic formulas—When, to whom and how long: After more than 15 years we know the right indication! Allergy 2004, 59, 45–52. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Guo, M. Processing technology for infant formula. In Human Milk Biochemistry and Infant Formula Manufacturing Technology; Woodhead Publishing Limited: Sawston, UK, 2014. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Chen, F.; Liu, K.; Zhu, T. Milk processing as a tool to reduce cow’s milk allergenicity: A mini-review. Dairy Sci. Technol. 2013, 93, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Fischer, J. Keeping enzymes kosher: Sacred and secular biotech production. EMBO Rep. 2015, 16, 681–684. [Google Scholar] [CrossRef] [Green Version]
- Morisawa, Y.; Kitamura, A.; Ujihara, T.; Zushi, N.; Kuzume, K.; Shimanouchi, Y.; Tamura, S.; Wakiguchi, H.; Saito, H.; Matsumoto, K. Effect of heat treatment and enzymatic digestion on the B cell epitopes of cow’s milk proteins. Clin. Exp. Allergy 2009, 39, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Bhatia, J.; Shamir, R.; Agostoni, C.; Turck, D.; Staiano, A.; Szajewska, H. Hydrolyzed formulas for allergy prevention. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 549–552. [Google Scholar] [CrossRef]
- Lowe, A.J.; Dharmage, S.C.; Allen, K.J.; Tang, M.L.; Hill, D.J. The role of partially hydrolyzed whey formula for the prevention of allergic disease: Evidence and gaps. Expert Rev. Clin. Immunol. 2013, 9, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Meulenbroek, L.A.P.M.; Den Hartog Jager, C.F.; Lebens, A.F.M.; Knulst, A.C.; Bruijnzeel-Koomen, C.A.F.M.; Garssen, J.; Knippels, L.M.J.; Van Hoffen, E. Characterization of T cell epitopes in bovine α-lactalbumin. Int. Arch. Allergy Immunol. 2014, 163, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Schünemann, H.J.; Brozek, J.; Restani, P.; Beyer, K.; Troncone, R.; Martelli, A.; Terracciano, L.; Bahna, S.L.; Rancé, F.; et al. Diagnosis and rationale for action against Cow’s milk allergy (DRACMA): A summary report. J. Allergy Clin. Immunol. 2010, 126, 1119–1128. [Google Scholar] [CrossRef]
- Verduci, E.; D’Elios, S.; Cerrato, L.; Comberiati, P.; Calvani, M.; Palazzo, S.; Martelli, A.; Landi, M.; Trikamjee, T.; Peroni, D.G. Cow’s Milk Substitutes for Children: Nutritional Aspects of Milk from Different Mammalian Species, Special Formula and Plant-Based Beverages. Nutrients 2019, 11, 1739. [Google Scholar] [CrossRef] [Green Version]
- Vandenplas, Y.; Munasir, Z.; Hegar, B.; Kumarawati, D.; Suryawan, A.; Kadim, M.; Djais, J.T.; Basrowi, R.W.; Krisnamurti, D. A perspective on partially hydrolyzed protein infant formula in nonexclusively breastfed infants. Korean J. Pediatr. 2019, 62, 149–154. [Google Scholar] [CrossRef]
- Niggemann, B.; Binder, C.; Klettke, U.; Wahn, U. In Vivo and In Vitro studies on the residual allergenicity of partially hydrolysed infant formulae. Acta Paediatr. Nurtur. Child 1999, 88, 394–398. [Google Scholar] [CrossRef]
- Caffarelli, C.; Plebani, A.; Poiesi, C.; Petroccione, T.; Spattini, A.; Cavagni, G. Determination of allergenicity to three cow’s milk hydrolysates and an amino acidderived formula in children with cow’s milk allergy. Clin. Exp. Allergy 2002, 32, 74–79. [Google Scholar] [CrossRef]
- Van Esch, B.C.A.M.; van Bilsen, J.H.M.; Jeurink, P.V.; Garssen, J.; Penninks, A.H.; Smit, J.J.; Pieters, R.H.H.; Knippels, L.M.J. Interlaboratory evaluation of a cow’s milk allergy mouse model to assess the allergenicity of hydrolysed cow’s milk based infant formulas. Toxicol. Lett. 2013, 220, 95–102. [Google Scholar] [CrossRef]
- Van Esch, B.C.A.M.; van Bilsen, J.H.M.; Gros-van Hest, M.; Kleinjans, L.; Belzer, C.; Jeurink, P.V.; Garssen, J.; Smit, J.J.; Pieters, R.H.H.; Knippels, L.M.J. A multi-center assessment to compare residual allergenicity of partial hydrolyzed whey proteins in a murine model for cow’s milk allergy—Comparison to the single parameter guinea pig model. Toxicol. Lett. 2020, 333, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, R. Animal models in food allergy: Assessment of allergenicity and preventive activity of infant formulas. Toxicol. Lett. 2003, 141, 303–309. [Google Scholar] [CrossRef]
- Jensen, L.H.; Larsen, J.M.; Madsen, C.B.; Laursen, R.R.; Jacobsen, L.N.; Bøgh, K.L. Preclinical Brown Norway Rat Models for the Assessment of Infant Formulas in the Prevention and Treatment of Cow’s Milk Allergy. Int. Arch. Allergy Immunol. 2019, 178, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, B.; Quarcoo, D.; Buhner, S.; Reese, G.; Vieths, S.; Hamelmann, E. Development of an animal model to evaluate the allergenicity of food allergens. Int. Arch. Allergy Immunol. 2014, 164, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Bøgh, K.L.; Barkholt, V.; Madsen, C.B. Characterization of the Immunogenicity and Allergenicity of Two Cow’s Milk Hydrolysates—A Study in Brown Norway Rats. Scand. J. Immunol. 2015, 81, 274–283. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Wang, Z.; Yang, H.; Luo, X.; Sun, J.; Yang, M.; Shi, X.; Yue, X.; Zheng, Y. Evaluation of allergenicity of cow milk treated with enzymatic hydrolysis through a mouse model of allergy. J. Dairy Sci. 2022, 105, 1039–1050. [Google Scholar] [CrossRef]
- Van Esch, B.C.A.M.; Knipping, K.; Jeurink, P.; van der Heide, S.; Dubois, A.E.J.; Willemsen, L.E.M.; Garssen, J.; Knippels, L.M.J. In vivo and in vitro evaluation of the residual allergenicity of partially hydrolysed infant formulas. Toxicol. Lett. 2011, 201, 264–269. [Google Scholar] [CrossRef]
- Kiewiet, M.B.G.; van Esch, B.C.A.M.; Garssen, J.; Faas, M.M.; de Vos, P. Partially hydrolyzed whey proteins prevent clinical symptoms in a cow’s milk allergy mouse model and enhance regulatory T and B cell frequencies. Mol. Nutr. Food Res. 2017, 61, 1700340. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Hauser, B.; den Borre, C.; Sacre, L.; Dab, I. Effect of a whey hydrolysate prophylaxis of atopic disease. Ann. Allergy 1992, 68, 419–424. [Google Scholar]
- Chandra, R.K. Five-year follow up of high-risk infants with family history of allergy exclusively breast-fed or fed partial whey hydrolysate, soy and conventional cow’s milk formulas. Nutr. Res. 1998, 18, 1395–1411. [Google Scholar] [CrossRef]
- Von Berg, A.; Filipiak-Pittroff, B.; Krämer, U.; Link, E.; Heinrich, J.; Koletzko, S.; Grübl, A.; Hoffmann, U.; Beckmann, C.; Reinhardt, D.; et al. The German Infant Nutritional Intervention Study (GINI) for the preventive effect of hydrolysed infant formulas in infants at high risk for allergic diseases. Design and selected results. Allergol. Sel. 2017, 1, 28–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, A.J.; Hosking, C.S.; Bennett, C.M.; Allen, K.J.; Axelrad, C.; Carlin, J.B.; Abramson, M.J.; Dharmage, S.C.; Hill, D.J. Effect of a partially hydrolyzed whey infant formula at weaning on risk of allergic disease in high-risk children: A randomized controlled trial. J. Allergy Clin. Immunol. 2011, 128, 360–365.e4. [Google Scholar] [CrossRef] [PubMed]
- De Silva, D.; Halken, S.; Singh, C.; Muraro, A.; Angier, E.; Arasi, S.; Arshad, H.; Beyer, K.; Boyle, R.; du Toit, G.; et al. Preventing food allergy in infancy and childhood: Systematic review of randomised controlled trials. Pediatr. Allergy Immunol. 2020, 31, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Graversen, K.B.; Larsen, J.M.; Pedersen, S.S.; Sørensen, L.V.; Christoffersen, H.F.; Jacobsen, L.N.; Halken, S.; Licht, T.R.; Bahl, M.I.; Bøgh, K.L. Partially Hydrolysed Whey Has Superior Allergy Preventive Capacity Compared to Intact Whey Regardless of Amoxicillin Administration in Brown Norway Rats. Front. Immunol. 2021, 12, 705543. [Google Scholar] [CrossRef] [PubMed]
- Fritsché, R.; Pahud, J.J.; Pecquet, S.; Pfeifer, A. Induction of systemic immunologic tolerance to β-lactoglobulin by oral administration of a whey protein hydrolysate. J. Allergy Clin. Immunol. 1997, 100, 266–273. [Google Scholar] [CrossRef]
- Chikhi, A.; Elmecherfi, K.E.; Bernard, H.; Cortes-Perez, N.; Kheroua, O.; Saidi, D.; Adel-Patient, K. Evaluation of the efficiency of hydrolyzed whey formula to prevent cow’s milk allergy in the BALB/c mouse model. Pediatr. Allergy Immunol. 2019, 30, 370–377. [Google Scholar] [CrossRef]
- Lambers, T.T.; Gloerich, J.; van Hoffen, E.; Alkema, W.; Hondmann, D.H.; van Toll, E.A.F. Clustering analyses in peptidomics revealed that peptide profiles of infant formulae are descriptive. Food Sci. Nutr. 2015, 3, 81–90. [Google Scholar] [CrossRef]
- Rosendal, A.; Barkholt, V. Detection of Potentially Allergenic Material in 12 Hydrolyzed Milk Formulas. J. Dairy Sci. 2000, 83, 2200–2210. [Google Scholar] [CrossRef]
- Gomes-Santos, A.C.; Fonseca, R.C.; Lemos, L.; Reis, D.S.; Moreira, T.G.; Souza, A.L.; Silva, M.R.; Silvestre, M.P.C.; Cara, D.C.; Faria, A.M.C. Hydrolyzed whey protein prevents the development of food allergy to β-lactoglobulin in sensitized mice. Cell Immunol. 2015, 298, 47–53. [Google Scholar] [CrossRef]
- Van Beresteijn, E.C.H.; Meijer, R.J.G.M.; Schmidt, D.G. Residual antigenicity of hypoallergenic infant formulas and the occurrence of milk-specific IgE antibodies in patients with clinical allergy. J. Allergy Clin. Immunol. 1995, 96, 365–374. [Google Scholar] [CrossRef]
- Schwartz, R.H.; Amonette, M.S. Cow milk protein hydrolysate infant formulas not always “hypoallergenic”. J. Pediatr. 1991, 119, 839–840. [Google Scholar] [CrossRef]
- Miraglia Del Giudice, M.; D’Auria, E.; Peroni, D.; Palazzo, S.; Radaelli, G.; Comberiati, P.; Galdo, F.; Maiello, N.; Riva, E. Flavor, relative palatability and components of cow’s milk hydrolysed formulas and amino acid-based formula Allergology and Immunology. Ital. J. Pediatr. 2015, 41, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Jiang, D.; Peterson, D.G. Identification of bitter peptides in whey protein hydrolysate. J. Agric. Food Chem. 2014, 62, 5719–5725. [Google Scholar] [CrossRef] [PubMed]
- Granier, A.; Goulet, O.; Hoarau, C. Fermentation products: Immunological effects on human and animal models. Pediatr. Res. 2013, 74, 238–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pescuma, M.; Hébert, E.M.; Mozzi, F.; De Valdez, G.F. Hydrolysis of whey proteins by Lactobacillus acidophilus, Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus grown in a chemically defined medium. J. Appl. Microbiol. 2007, 103, 1738–1746. [Google Scholar] [CrossRef] [PubMed]
- El-Ghaish, S.; Rabesona, H.; Choiset, Y.; Sitohy, M.; Haertlé, T.; Chobert, J.M. Proteolysis by Lactobacillus fermentum IFO3956 isolated from Egyptian milk products decreases immuno-reactivity of αs1-casein. J. Dairy Res. 2011, 78, 203–210. [Google Scholar] [CrossRef]
- Ahmadova, A.; El-Ghaish, S.; Choiset, Y.; Rabesona, H.; Drouet, M.; Chobert, J.M.; Kuliev, A.A.; Haertle, T. Modification of ige binding to β- and αs1-caseins by proteolytic activity of lactobacillus helveticus a75. J. Food Biochem. 2013, 37, 491–500. [Google Scholar] [CrossRef]
- Pescuma, M.; Hébert, E.M.; Rabesona, H.; Drouet, M.; Choiset, Y.; Haertlé, T.; Mozzi, F.; De Valdez, G.F.; Chobert, J.M. Proteolytic action of Lactobacillus delbrueckii subsp. bulgaricus CRL 656 reduces antigenic response to bovine β-lactoglobulin. Food Chem. 2011, 127, 487–492. [Google Scholar] [CrossRef]
- Ménard, S.; Candalh, C.; Ben, A.M.; Rakotobe, S.; Gaboriau-Routhiau, V.; Cerf-Bensussan, N.; Heyman, M. Stimulation of immunity without alteration of oral tolerance in mice fed with heat-treated fermented infant formula. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 451–458. [Google Scholar] [CrossRef]
- Morisset, M.; Aubert-Jacquin, C.; Soulaines, P.; Moneret-Vautrin, D.A.; Dupont, C. A non-hydrolyzed, fermented milk formula reduces digestive and respiratory events in infants at high risk of allergy. Eur. J. Clin. Nutr. 2011, 65, 175–183. [Google Scholar] [CrossRef]
- Szajewska, H.; Skórka, A.; Pieścik-Lech, M. Fermented infant formulas without live bacteria: A systematic review. Eur. J. Pediatr. 2015, 174, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Nasirpour, A.; Scher, J.; Desobry, S. Baby foods: Formulations and interactions (a review). Crit. Rev. Food Sci. Nutr. 2006, 46, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Pischetsrieder, M.; Henle, T. Glycation products in infant formulas: Chemical, analytical and physiological aspects. Amino Acids 2012, 42, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Rudloff, S.; Lonnerdal, B. Solubility and Digestability of Milk Proteins in Infant Formulas Exposed to Different Heat Treatments. J. Pediatr. Gastroenterol. Nutr. 1992, 15, 25–33. [Google Scholar] [CrossRef]
- De Wit, J.N. Nutritional and Functional Characteristics of Whey Proteins in Food Products. J. Dairy Sci. 1998, 81, 597–608. [Google Scholar] [CrossRef]
- De Witt, J.N.; Klarenbeek, G. Effects of Various Heat Treatments on Structure and Solubility of Whey Proteins. J. Dairy Sci. 1984, 67, 2701–2710. [Google Scholar] [CrossRef]
- Donato, L.; Guyomarc’h, F. Formation and properties of the whey protein/κ-casein complexes in heated skim milk—A review. Dairy Sci. Technol. 2009, 89, 3–29. [Google Scholar] [CrossRef]
- Van Lieshout, G.A.A.; Lambers, T.T.; Bragt, M.C.E.; Hettinga, K.A. How processing may affect milk protein digestion and overall physiological outcomes: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2422–2445. [Google Scholar] [CrossRef] [Green Version]
- Guyomarc’h, F.; Law, A.J.R.; Dalgleish, D.G. Formation of soluble and micelle-bound protein aggregates in heated milk. J. Agric. Food Chem. 2003, 51, 4652–4660. [Google Scholar] [CrossRef]
- Lowe, E.K.; Anema, S.G.; Bienvenue, A.; Roland, M.J.; Creamer, L.K.; Jiménez-Flores, R. Heat-induced redistribution of disultide bonds in milk proteins. 2. Disulfide bonding patterns between bovine β-lactoglobulin and κ-casein. J. Agric. Food Chem. 2004, 52, 7669–7680. [Google Scholar] [CrossRef]
- Creamer, L.K.; Bienvenue, A.; Nilsson, H.; Paulsson, M.; Van Wanroij, M.; Lowe, E.K.; Anema, S.G.; Boland, M.J.; Jiménez-Flores, R. Heat-induced redistribution of disulfide bonds in milk proteins. 1. Bovine β-lactoglobulin. J. Agric. Food Chem. 2004, 52, 7660–7668. [Google Scholar] [CrossRef] [PubMed]
- Krishna, T.C.; Najda, A.; Bains, A.; Tosif, M.M.; Papliński, R.; Kapłan, M.; Chawla, P. Influence of ultra-heat treatment on properties of milk proteins. Polymers 2021, 13, 3164. [Google Scholar] [CrossRef] [PubMed]
- Corredig, M.; Dalgleish, D.G. Effect of temperature and pH on the interactions of whey proteins with casein micelles in skim milk. Food Res. Int. 1996, 29, 49–55. [Google Scholar] [CrossRef]
- Dalgleish, D.G.; Corredig, M. The structure of the casein micelle of milk and its changes during processing. Annu. Rev. Food Sci. Technol. 2012, 3, 449–467. [Google Scholar] [CrossRef]
- Singh, H.; Latham, J.M. Heat stability of milk: Aggregation and dissociation of protein at ultra-high temperatures. Int. Dairy J. 1993, 3, 225–237. [Google Scholar] [CrossRef]
- Donovan, M.; Mulvihill, D.M. Thermal Denaturation and Aggregation of Whey Proteins. Agric. Food Dev. Auth. 1987, 11, 87–100. [Google Scholar]
- Chaplin, L.C.; Lyster, R.L. Irreversible heat denaturation of bovine alpha-lactalbumin. J. Dairy Res. 1986, 53, 249–258. [Google Scholar] [CrossRef]
- Nguyen, N.H.A.; Streicher, C.; Anema, S.G. The effect of thiol reagents on the denaturation of the whey protein in milk and whey protein concentrate solutions. Int. Dairy J. 2018, 85, 285–293. [Google Scholar] [CrossRef]
- Abbring, S.; Xiong, L.; Diks, M.A.P.; Baars, T.; Garssen, J.; Hettinga, K.; Van Esch, B.C.A.M. Loss of allergy-protective capacity of raw cow’s milk after heat treatment coincides with loss of immunologically active whey proteins. Food Funct. 2020, 11, 4982–4993. [Google Scholar] [CrossRef]
- Ye, M.P.; Zhou, R.; Shi, Y.R.; Chen, H.C.; Du, Y. Effects of heating on the secondary structure of proteins in milk powders using mid-infrared spectroscopy. J. Dairy Sci. 2017, 100, 89–95. [Google Scholar] [CrossRef]
- Crowley, S.V.; Dowling, A.P.; Caldeo, V.; Kelly, A.L.; O’Mahony, J.A. Impact of α-lactalbumin:β-lactoglobulin ratio on the heat stability of model infant milk formula protein systems. Food Chem. 2016, 194, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Halabi, A.; Deglaire, A.; Hamon, P.; Bouhallab, S.; Dupont, D.; Croguennec, T. Kinetics of heat-induced denaturation of proteins in model infant milk formulas as a function of whey protein composition. Food Chem. 2020, 302, 125296. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.; Boutrou, R.; Menard, O.; Jardin, J.; Tanguy, G.; Schuck, P.; Haab, B.B.; Leonil, J. Heat treatment of milk during powder manufacture increases casein resistance to simulated infant digestion. Food Dig. 2010, 1, 28–39. [Google Scholar] [CrossRef]
- He, T.; Rombouts, W.; Einerhand, A.W.C.; Hotrum, N.; van de Velde, F. Gastric protein digestion of goat and cow milk infant formula and human milk under simulated infant conditions. Int. J. Food Sci. Nutr. 2021, 73, 28–38. [Google Scholar] [CrossRef]
- Halabi, A.; Croguennec, T.; Bouhallab, S.; Dupont, D.; Deglaire, A. Modification of protein structures by altering the whey protein profile and heat treatment affects: In vitro static digestion of model infant milk formulas. Food Funct. 2020, 11, 6933–6945. [Google Scholar] [CrossRef]
- Rinaldi, L.; Gauthier, S.F.; Britten, M.; Turgeon, S.L. Invitro gastrointestinal digestion of liquid and semi-liquid dairy matrixes. LWT-Food Sci. Technol. 2014, 57, 99–105. [Google Scholar] [CrossRef]
- Barbé, F.; Ménard, O.; Le Gouar, Y.; Buffière, C.; Famelart, M.H.; Laroche, B.; Le Feunteun, S.; Dupont, D.; Rémond, D. The heat treatment and the gelation are strong determinants of the kinetics of milk proteins digestion and of the peripheral availability of amino acids. Food Chem. 2013, 136, 1203–1212. [Google Scholar] [CrossRef]
- Peram, M.R.; Loveday, S.M.; Ye, A.; Singh, H. In Vitro gastric digestion of heat-induced aggregates of β-lactoglobulin. J. Dairy Sci. 2013, 96, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Meltretter, J.; Seeber, S.; Humeny, A.; Becker, C.M.; Pischetsrieder, M. Site-specific formation of maillard, oxidation, and condensation products from whey proteins during reaction with lactose. J. Agric. Food Chem. 2007, 55, 6096–6103. [Google Scholar] [CrossRef]
- Chen, Z.; Leinisch, F.; Greco, I.; Zhang, W.; Shu, N.; Chuang, C.Y.; Lund, M.N.; Davies, M.J. Characterisation and quantification of protein oxidative modifications and amino acid racemisation in powdered infant milk formula. Free Radic. Res. 2019, 53, 68–81. [Google Scholar] [CrossRef]
- Fenaille, F.; Parisod, V.; Tabet, J.C.; Guy, P.A. Carbonylation of milk powder proteins as a consequence of processing conditions. Proteomics 2005, 5, 3097–3104. [Google Scholar] [CrossRef]
- Fenaille, F.; Parisod, V.; Visani, P.; Populaire, S.; Tabet, J.C.; Guy, P.A. Modifications of milk constituents during processing: A preliminary benchmarking study. Int. Dairy J. 2006, 16, 728–739. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Scheidegger, D.; Radici, P.M.; Vergara-Roig, V.A.; Bosio, N.S.; Pesce, S.F.; Pecora, R.P.; Romano, J.C.P.; Kivatinitz, S.C. Evaluation of milk powder quality by protein oxidative modifications. J. Dairy Sci. 2013, 96, 3414–3423. [Google Scholar] [CrossRef] [PubMed]
- Giblin, L.; Yalçın, A.S.; Biçim, G.; Krämer, A.C.; Chen, Z.; Callanan, M.J.; Arranz, E.; Davies, M.J. Whey proteins: Targets of oxidation, or mediators of redox protection. Free Radic. Res. 2019, 53, 1136–1152. [Google Scholar] [CrossRef]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Li, C.; Ullah, N.; Cao, J.; Lan, Y.; Ge, W.; Hackman, R.M.; Li, Z.; Chen, L. Susceptibility of whey protein isolate to oxidation and changes in physicochemical, structural, and digestibility characteristics. J. Dairy Sci. 2015, 98, 7602–7613. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.H.; Zhao, X.H. In vitro digestibility and rheological properties of caseinates treated by an oxidative system containing horseradish peroxidase, glucose oxidase and glucose. Int. Dairy J. 2012, 27, 47–52. [Google Scholar] [CrossRef]
- Meyer, B.; Baum, F.; Vollmer, G.; Pischetsrieder, M. Distribution of protein oxidation products in the proteome of thermally processed milk. J. Agric. Food Chem. 2012, 60, 7306–7311. [Google Scholar] [CrossRef]
- Gill, V.; Kumar, V.; Singh, K.; Kumar, A.; Kim, J.J. Advanced glycation end products (AGEs) may be a striking link between modern diet and health. Biomolecules 2019, 9, 888. [Google Scholar] [CrossRef] [Green Version]
- Marvin, L.F.; Parisod, V.; Fay, L.B.; Guy, P.A. Characterization of lactosylated proteins of infant formula powders using two-dimensional gel electrophoresis and nanoelectrospray mass spectrometry. Electrophoresis 2002, 23, 2505–2512. [Google Scholar] [CrossRef]
- Milkovska-Stamenova, S.; Hoffmann, R. Influence of storage and heating on protein glycation levels of processed lactose-free and regular bovine milk products. Food Chem. 2017, 221, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Wölk, M.; Milkovska-Stamenova, S.; Hoffmann, R. Comprehensive profiling of the native and modified peptidomes of raw bovine milk and processed milk products. Foods 2020, 9, 1841. [Google Scholar] [CrossRef] [PubMed]
- Zenker, H.E.; Van Lieshout, G.A.A.; Van Gool, M.P.; Bragt, M.C.E.; Hettinga, K.A. Lysine blockage of milk proteins in infant formula impairs overall protein digestibility and peptide release. Food Funct. 2020, 11, 358–369. [Google Scholar] [CrossRef] [Green Version]
- Sarriá, B.; López-Fandiño, R.; Vaquero, M.P. Protein nutritive utilization in rats fed powder and liquid infant formulas/Utilización nutritiva de la proteína en ratas alimentadas con formulas infantiles en polvo y líquidas. Food Sci. Technol. Int. 2000, 6, 9–16. [Google Scholar] [CrossRef]
- Contreras-Calderón, J.; Guerra-Hernández, E.; García-Villanova, B. Indicators of non-enzymatic browning in the evaluation of heat damage of ingredient proteins used in manufactured infant formulas. Eur. Food Res. Technol. 2008, 227, 117–124. [Google Scholar] [CrossRef]
- Meltretter, J.; Birlouez-Aragon, I.; Becker, C.M.; Pischetsrieder, M. Assessment of heat treatment of dairy products by MALDI-TOF-MS. Mol. Nutr. Food Res. 2009, 53, 1487–1495. [Google Scholar] [CrossRef]
- Wada, Y.; Lönnerdal, B. Effects of different industrial heating processes of milk on site-specific protein modifications and their relationship to in vitro and in vivo digestibility. J. Agric. Food Chem. 2014, 62, 4175–4185. [Google Scholar] [CrossRef]
- Lee, H.M.; Yang, S.Y.; Han, J.; Kim, Y.K.; Kim, Y.J.; Rhee, M.S.; Lee, K.W. Optimization of spray drying parameters and food additives to reduce glycation using response surface methodology in powdered infant formulas. Food Sci. Biotechnol. 2019, 28, 769–777. [Google Scholar] [CrossRef]
- Cattaneo, S.; Masotti, F.; Pellegrino, L. Liquid infant formulas: Technological tools for limiting heat damage. J. Agric. Food Chem. 2009, 57, 10689–10694. [Google Scholar] [CrossRef]
- Oh, N.S.; Young Lee, J.; Lee, H.A.; Joung, J.Y.; Shin, Y.K.; Kim, S.H.; Kim, Y.; Lee, K.W. Chemical characteristics and enhanced hepatoprotective activities of Maillard reaction products derived from milk protein-sugar system. J. Dairy Sci. 2016, 99, 947–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Luo, Y.; Feng, L. Effects of Maillard reaction conditions on the antigenicity of α-lactalbumin and β-lactoglobulin in whey protein conjugated with maltose. Eur. Food Res. Technol. 2011, 233, 387–394. [Google Scholar] [CrossRef]
- Heppell, L.M.J.; Cant, A.J.; Kilshaw, P.J. Reduction in the antigenicity of whey proteins by heat treatment: A possible strategy for producing a hypoallergenic infant milk formula. Br. J. Nutr. 1984, 51, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleber, N.; Krause, I.; Illgner, S.; Hinrichs, J. The antigenic response of β-lactoglobulin is modulated by thermally induced aggregation. Eur. Food Res. Technol. 2004, 219, 105–110. [Google Scholar] [CrossRef]
- Ehn, B.M.; Ekstrand, B.; Bengtsson, U.; Ahlstedt, S. Modification of IgE Binding during Heat Processing of the Cow’s Milk Allergen β-Lactoglobulin. J. Agric. Food Chem. 2004, 52, 1398–1403. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Zheng, Z.; Zheng, H. Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin in whey protein isolate. Food Agric. Immunol. 2009, 20, 195–206. [Google Scholar] [CrossRef]
- Xu, Q.; Shi, J.; Yao, M.; Jiang, M.; Luo, Y. Effects of heat treatment on the antigenicity of four milk proteins in milk protein concentrates. Food Agric. Immunol. 2016, 27, 401–413. [Google Scholar] [CrossRef]
- Taheri-Kafrani, A.; Gaudin, J.C.; Rabesona, H.; Nioi, C.; Agarwal, D.; Drouet, M.; Chobert, J.M.; Bordbar, A.K.; Haertle, T. Effects of heating and glycation of β-lactoglobulin on its recognition by ige of sera from cow milk allergy patients. J. Agric. Food Chem. 2009, 57, 4974–4982. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Berin, M.C.; Arnaboldi, P.; Escalante, C.R.; Dahan, S.; Rauch, J.; Jensen-Jarolim, E.; Mayer, L. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer’s patches. Allergy Eur. J. Allergy Clin. Immunol. 2008, 63, 882–890. [Google Scholar] [CrossRef]
- Graversen, K.B.; Ballegaard, A.S.R.; Kræmer, L.H.; Hornslet, S.E.; Sørensen, L.V.; Christoffersen, H.F.; Jacobsen, L.N.; Untersmayr, E.; Smit, J.J.; Bøgh, K.L. Cow’s milk allergy prevention and treatment by heat-treated whey—A study in Brown Norway rats. Clin. Exp. Allergy 2020, 50, 708–721. [Google Scholar] [CrossRef]
- Kleber, N.; Maier, S.; Hinrichs, J. Antigenic response of bovine β-lactoglobulin influenced by ultra-high pressure treatment and temperature. Innov. Food Sci. Emerg. Technol. 2007, 8, 39–45. [Google Scholar] [CrossRef]
- Zhong, J.; Liu, C.; Liu, W.; Cai, X.; Tu, Z.; Wan, J. Effect of dynamic high-pressure microfluidization at different temperatures on the antigenic response of bovine β-lactoglobulin. Eur. Food Res. Technol. 2011, 233, 95–102. [Google Scholar] [CrossRef]
- Chicón, R.; Belloque, J.; Alonso, E.; López-Fandiño, R. Immunoreactivity and digestibility of high-pressure-treated whey proteins. Int. Dairy J. 2008, 18, 367–376. [Google Scholar] [CrossRef]
- Boughellout, H.; Choiset, Y.; Rabesona, H.; Chobert, J.M.; Haertle, T.; Mounir, S.; Allaf, K.; Zidoune, M.N. Effect of instant controlled pressure drop (DIC) treatment on milk protein’s immunoreactivity. Food Agric. Immunol. 2015, 26, 71–81. [Google Scholar] [CrossRef]
- Odueke, O.B.; Farag, K.W.; Baines, R.N.; Chadd, S.A. Irradiation Applications in Dairy Products: A Review. Food Bioprocess Technol. 2016, 9, 751–767. [Google Scholar] [CrossRef]
- Tammineedi, C.V.R.K.; Choudhary, R.; Perez-Alvarado, G.C.; Watson, D.G. Determining the effect of UV-C, high intensity ultrasound and nonthermal atmospheric plasma treatments on reducing the allergenicity of α-casein and whey proteins. LWT—Food Sci. Technol. 2013, 54, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Zheng, Y.; Liu, Z.; Deng, Y.; Zhao, Y. Structure and IgE-binding properties of α-casein treated by high hydrostatic pressure, UV-C, and far-IR radiations. Food Chem. 2016, 204, 46–55. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, J.H.; Yook, H.S.; Kang, K.O.; Lee, S.Y.; Hwang, H.J.; Byun, M.W. Effects of gamma radiation on the allergenic and antigenic properties of milk proteins. J. Food Prot. 2001, 64, 272–276. [Google Scholar] [CrossRef]
- Stanic-Vucinic, D.; Stojadinovic, M.; Atanaskovic-Markovic, M.; Ognjenovic, J.; Grönlund, H.; van Hage, M.; Lantto, R.; Sancho, A.I.; Velickovic, T.C. Structural changes and allergenic properties of β-lactoglobulin upon exposure to high-intensity ultrasound. Mol. Nutr. Food Res. 2012, 56, 1894–1905. [Google Scholar] [CrossRef]
- Tammineedi, C.V.R.K.; Choudhary, R. Recent Advances in Processing for Reducing Dairy and Food Allergenicity. Int. J. Food Sci. Nutr. Eng. 2014, 4, 36–42. [Google Scholar]
- Xiang, B.Y.; Ngadi, M.O.; Ochoa-Martinez, L.A.; Simpson, M.V. Pulsed Electric Field-Induced Structural Modification of Whey Protein Isolate. Food Bioprocess Technol. 2011, 4, 1341–1348. [Google Scholar] [CrossRef]
- Meyer, R.; Groetch, M.; Venter, C. When Should Infants with Cow’s Milk Protein Allergy Use an Amino Acid Formula? A Practical Guide. J. Allergy Clin. Immunol. Pract. 2017, 6, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Vanderhoof, J.A. In time: Misuse and overuse of amino acid formulas in cow milk allergy. Rev. Paul. Pediatr. 2015, 33, 379–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicherer, S.H.; Noone, S.A.; Koerner, C.B.; Christie, L.; Burks, A.W.; Sampson, H.A. Hypoallergenicity and efficacy of an amino acid-based formula in children with cow’s milk and multiple food hypersensitivities. J. Pediatr. 2001, 138, 688–693. [Google Scholar] [CrossRef]
- Nowak-Wagrzyn, A.; Czerkies, L.A.; Collins, B.; Saavedra, J.M. Evaluation of hypoallergenicity of a new, amino acid-based formula. Clin. Pediatr. 2015, 54, 264–272. [Google Scholar] [CrossRef]
- Canani, R.B.; Nocerino, R.; Frediani, T.; Lucarelli, S.; Di Scala, C.; Varin, E.; Leone, L.; Muraro, A.; Agostoni, C. Amino acid-based formula in cow’s milk allergy: Long-term effects on body growth and protein metabolism. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 632–638. [Google Scholar] [CrossRef]
- Corkins, M.; Czerkies, L.A.; Storm, H.M.; Sun, S.; Saavedra, J.M. Assessment of Growth of Infants Fed an Amino Acid-Based Formula. Clin. Med. Insights Pediatr. 2016, 10, CMPed.S33071. [Google Scholar] [CrossRef] [Green Version]
- Vandenplas, Y.; Dupont, C.; Eigenmann, P.; Heine, R.G.; Høst, A.; Järvi, A.; Kuitunen, M.; Mukherjee, R.; Ribes-Koninckx, C.; Szajewska, H.; et al. Growth in Infants with Cow’s Milk Protein Allergy Fed an Amino AcidBased Formula. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 392–402. [Google Scholar] [CrossRef]
- Panel, E.; Nda, A. Scientific Opinion on the suitability of goat milk protein as a source of protein in infant formulae and in follow-on formulae. EFSA J. 2012, 10, 2603. [Google Scholar]
- Faye, B.; Konuspayeva, G. The sustainability challenge to the dairy sector—The growing importance of non-cattle milk production worldwide. Int. Dairy J. 2012, 24, 50–56. [Google Scholar] [CrossRef]
- Hackmann, T.J.; Spain, J.N. Invited review: Ruminant ecology and evolution: Perspectives useful to ruminant livestock research and production. J. Dairy Sci. 2010, 93, 1320–1334. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.A.; Ciani, E.; Faye, B. Old World camels in a modern world—A balancing act between conservation and genetic improvement. Anim. Genet. 2019, 50, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Alberto, F.J.; Boyer, F.; Orozco-Terwengel, P.; Streeter, I.; Servin, B.; De Villemereuil, P.; Benjelloun, B.; Librado, P.; Biscarini, F.; Colli, L.; et al. Convergent genomic signatures of domestication in sheep and goats. Nat. Commun. 2018, 9, 813. [Google Scholar] [CrossRef]
- Brosnahan, M.M. Genetics, Evolution, and Physiology of Donkeys and Mules. Vet. Clin. N. Am. Equine Pract. 2019, 35, 457–467. [Google Scholar] [CrossRef]
- Maryniak, N.Z.; Hansen, E.B.; Ballegaard, A.-S.R.; Sancho, A.I.; Bøgh, K.L. Comparison of the allergenicity and immunogenicity of Camel and cow’s milk—A study in brown Norway rats. Nutrients 2018, 10, 1903. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.C.; Ribeiro, S.D.A. Specialty products made from goat milk. Small Rumin. Res. 2010, 89, 226–234. [Google Scholar] [CrossRef]
- Prosser, C.G. Compositional and functional characteristics of goat milk and relevance as a base for infant formula. J. Food Sci. 2021, 86, 257–265. [Google Scholar] [CrossRef]
- Ceballos, L.S.; Morales, E.R.; de la Torre Adarve, G.; Castro, J.D.; Martínez, L.P.; Sampelayo, M.R.S. Composition of goat and cow milk produced under similar conditions and analyzed by identical methodology. J. Food Compos. Anal. 2009, 22, 322–329. [Google Scholar] [CrossRef]
- Park, Y.W. Hypo-allergenic and therapeutic significance of goat milk. Small Rumin. Res. 1994, 14, 151–159. [Google Scholar] [CrossRef]
- Restani, P. Goat milk allerginicity. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 323–324. [Google Scholar] [CrossRef]
- Jandal, J.M. Comparative aspects of goat and sheep milk. Small Rumin. Res. 1996, 22, 177–185. [Google Scholar] [CrossRef]
- Bellioni-Businco, B.; Paganelli, R.; Lucenti, P.; Giampietro, P.G.; Perborn, H.; Businco, L. Allergenicity of goat’s milk in children with cow’s milk allergy. J. Allergy Clin. Immunol. 1999, 103, 1191–1194. [Google Scholar] [CrossRef]
- Rodríguez del Río, P.; Sánchez-García, S.; Escudero, C.; Pastor-Vargas, C.; Sánchez Hernández, J.J.; Pérez-Rangel, I.; Ibáñez, M.D. Allergy to goat’s and sheep’s milk in a population of cow’s milk-allergic children treated with oral immunotherapy. Pediatr. Allergy Immunol. 2012, 23, 128–132. [Google Scholar] [CrossRef]
- Infante Pina, D.; Tormo Carnice, R.; Conde Zandueta, M. Use of goat’s milk in patients with cow’s milk allergy. An. Pediatr. 2003, 59, 138–142. [Google Scholar] [CrossRef]
- Ah-Leung, S.; Bernard, H.; Bidat, E.; Paty, E.; Rancé, F.; Scheinmann, P.; Wal, J.M. Allergy to goat and sheep milk without allergy to cow’s milk. Allergy Eur. J. Allergy Clin. Immunol. 2006, 61, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Caballer, H.; Gonz, R. Selective allergy to sheep’s and goat’s milk proteins. Allergol. Immunopathol. 2004, 32, 49–52. [Google Scholar]
- Tavares, B.; Pereira, C.; Rodrigues, F.; Loureiro, G.; Chieira, C. Goat’s milk allergy. Allergol. Immunopathol. 2007, 35, 113–116. [Google Scholar] [CrossRef]
- Hazebrouck, S.; Ah-Leung, S.; Bidat, E.; Paty, E.; Drumare, M.F.; Tilleul, S.; Adel-Patient, K.; Wal, J.M.; Bernard, H. Goat’s milk allergy without cow’s milk allergy: Suppression of non-cross-reactive epitopes on caprine β-casein. Clin. Exp. Allergy 2013, 44, 602–610. [Google Scholar] [CrossRef]
- Bernard, H.; Ah-Leung, S.; Tilleul, S.; Drumare, M.F.; Paty, E.; Bidat, E.; Wal, J.M.; Hazebrouck, S. Specificity of IgE antibodies from patients allergic to goat’s milk and tolerant to cow’s milk determined with plasmin-derived peptides of bovine and caprine β-caseins. Mol. Nutr. Food Res. 2012, 56, 1532–1540. [Google Scholar] [CrossRef]
- Lara-Villoslada, F.; Olivares, M.; Jiménez, J.; Boza, J.; Xaus, J. Goat milk is less immunogenic than cow milk in a murine model of atopy. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Ceballos, L.S.; Sampelayo, M.R.S.; Extremera, F.G.; Osorio, M.R. Evaluation of the allergenicity of goat milk, cow milk, and their lactosera in a guinea pig model. J. Dairy Sci. 2009, 92, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Shinde, A.K.; Singh, R. Sheep milk: A pertinent functional food. Small Rumin. Res. 2019, 181, 6–11. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Pimentel, T.C.; Ferrão, L.L.; Almada, C.N.; Santillo, A.; Albenzio, M.; Mollakhalili, N.; Mortazavian, A.M.; Nascimento, J.S.; Silva, M.C.; et al. Sheep Milk: Physicochemical Characteristics and Relevance for Functional Food Development. Compr. Rev. Food Sci. Food Saf. 2017, 16, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Ministry for Primary Industries. Labelling of Retail Ready Infant Formula and Formulated Sypplementary Food for Young Children; Ministry for Primary Industries: Wellington, New Zealand, 2018. [Google Scholar]
- Jianshui County Honghui Aquaculture Industry. Baby Formula Milk Powder Containing Desalted Sheep Whey Powder and Production Method of Baby Formula Milk Powder. Patent Application No.CN106615147A, 10 May 2017. [Google Scholar]
- Calvani, M.; Alessandri, C. Anaphylaxis to sheep’s milk cheese in a child unaffected by cow’s milk protein allergy. Eur. J. Pediatr. 1998, 157, 17–19. [Google Scholar] [CrossRef]
- Wüthrich, B.; Johansson, S.G.O. Allergy to cheese produced from sheep’s and goat’s milk but not to cheese produced from cow’s milk. J. Allergy Clin. Immunol. 1995, 96, 270–273. [Google Scholar] [CrossRef]
- Viñas, M.; Carnés, J.; López-Matas, M.A.; Hernández, N.; Castillo, M.J.; Ibero, M. Allergy to goat and sheep cheese with tolerance to cow’s milk and its derivatives. Allergol. Immunopathol. 2014, 42, 186–190. [Google Scholar] [CrossRef]
- Umpiérrez, A.; Quirce, S.; Marañón, F.; Cuesta, J.; García-Villamuza, Y.; Lahoz, C.; Sastre, J. Allergy to goat and sheep cheese with good tolerance to cow cheese. Clin. Exp. Allergy 1999, 29, 1064–1068. [Google Scholar] [CrossRef]
- Pazheri, F.; Melton, A.L.; Poptic, E.; Wallard, B. Allergy to Sheep Milk With Or Without Allergy To Cow Milk. J. Allergy Clin. Immumol. 2014, 133, AB199. [Google Scholar] [CrossRef]
- Barlowska, J.; Szwajkowska, M.; Litwińczuk, Z.; Król, J. Nutritional Value and Technological Suitability of Milk from Various Animal Species Used for Dairy Production. Compr. Rev. Food Sci. Food Saf. 2011, 10, 291–302. [Google Scholar] [CrossRef]
- Vincenzetti, S.; Cammertoni, N.; Rapaccetti, R.; Santini, G.; Klimanova, Y.; Zhang, J.-J.; Polidori, P. Nutraceutical and Functional Properties of Camelids’ Milk. Beverages 2022, 8, 12. [Google Scholar] [CrossRef]
- Hailu, Y.; Hansen, E.B.; Seifu, E.; Eshetu, M.; Ipsen, R.; Kappeler, S. Functional and technological properties of camel milk proteins: A review. J. Dairy Res. 2016, 83, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Sélo, I.; Clément, G.; Bernard, H.; Chatel, J.M.; Créminon, C.; Peltre, G.; Wal, J.M. Allergy to bovine β-lactoglobulin: Specificity of human IgE to tryptic peptides. Clin. Exp. Allergy 1999, 29, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.; Harbourne, N.; Oruna-Concha, M.J. Quantification of major camel milk proteins by capillary electrophoresis. Int. Dairy J. 2016, 58, 31–35. [Google Scholar] [CrossRef]
- Swelum, A.A.; El-Saadony, M.T.; Abdo, M.; Ombarak, R.A.; Hussein, E.O.S.; Suliman, G.; Alhimaidi, A.R.; Ammari, A.A.; Ba-Awadh, H.; Taha, A.E.; et al. Nutritional, antimicrobial and medicinal properties of Camel’s milk: A review. Saudi J. Biol. Sci. 2021, 28, 3126–3136. [Google Scholar] [CrossRef]
- Berhe, T.; Seifu, E.; Ipsen, R.; Kurtu, M.Y.; Hansen, E.B. Processing Challenges and Opportunities of Camel Dairy Products. Int. J. Food Sci. 2017, 2017, 9061757. [Google Scholar] [CrossRef] [Green Version]
- Fiocchi, A.; Bognanni, A.; Brożek, J.; Ebisawa, M.; Schünemann, H.; Ansotegui, I.J.; Arasi, S.; Assa’ad, A.H.; Bahna, S.L.; Canani, R.B.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines update—I—Plan and definitions. World Allergy Organ. J. 2022, 15, 100609. [Google Scholar] [CrossRef]
- Navarrete-Rodríguez, E.M.; Ríos-Villalobos, L.A.; Alcocer-Arreguín, C.R.; Del-Rio-Navarro, B.E.; Del Rio-Chivardi, J.M.; Saucedo-Ramírez, O.J.; Sienra-Monge, J.J.L.; Frias, R.V. Cross-over clinical trial for evaluating the safety of camel’s milk intake in patients who are allergic to cow’s milk protein. Allergol. Immunopathol. 2017, 46, 149–154. [Google Scholar] [CrossRef]
- Ehlayel, M.S.; Hazeima, K.A.; Al-Mesaifri, F.; Bener, A. Camel milk: An alternative for cow’s milk allergy in children. Allergy Asthma Proc. 2011, 32, 255–258. [Google Scholar] [CrossRef]
- Ehlayel, M.; Bener, A.; Abu Hazeima, K.; Al-Mesaifri, F. 1 Camel Milk Is a Safer Choice than Goat Milk for Feeding Children with Cow Milk Allergy. ISRN Allergy 2011, 2011, 391641. [Google Scholar] [CrossRef] [Green Version]
- Aburiziza, A.J. Cross reactivity between cow’s and camel’s milk in the infant population of Saudi Arabia. Curr. Pediatr. Res. 2020, 24, 175–179. [Google Scholar]
- Restani, P.; Beretta, B.; Fiocchi, A.; Ballabio, C.; Galli, C.L. Cross-reactivity between mammalian proteins. Ann. Allergy Asthma Immunol. 2002, 89, 11–15. [Google Scholar] [CrossRef]
- Restani, P.; Gaiaschi, A.; Plebani, A.; Beretta, B.; Cagavni, G.; Fiocchi, A.; Poiesi, C.; Velona, T.; Ugazio, A.G.; Galli, C.L. Cross-reactivity between milk proteins from different animal species. Clin. Exp. Allergy 1999, 29, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- El-Agamy, E.I.; Nawar, M.; Shamsia, S.M.; Awad, S.; Haenlein, G.F.W. Are camel milk proteins convenient to the nutrition of cow milk allergic children? Small Rumin. Res. 2009, 82, 1–6. [Google Scholar] [CrossRef]
- Vojdani, A.; Turnpaugh, C.; Vojdani, E. Immune reactivity against a variety of mammalian milks and plant-based milk substitutes. J. Dairy Res. 2018, 85, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Al-Hammadi, S.; El-Hassan, T.; Al-Reyami, L. Anaphylaxis to camel milk in an atopic child. Allergy 2010, 65, 1622–1623. [Google Scholar] [CrossRef]
- Aspri, M.; Economou, N.; Papademas, P. Donkey milk: An overview on functionality, technology, and future prospects. Food Rev. Int. 2017, 33, 316–333. [Google Scholar] [CrossRef]
- Polidori, P.; Beghelli, D.; Mariani, P.; Vincenzetti, S. Donkey milk production: State of the art. Ital. J. Anim. Sci. 2009, 8, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Martini, M.; Altomonte, I.; Licitra, R.; Salari, F. Nutritional and Nutraceutical Quality of Donkey Milk. J. Equine Vet. Sci. 2018, 65, 33–37. [Google Scholar] [CrossRef]
- Sarti, L.; Martini, M.; Brajon, G.; Barni, S.; Salari, F.; Altomonte, I.; Ragona, G.; Mori, F.; Pucci, N.; Muscas, G.; et al. Donkey’s Milk in the Management of Children with Cow’s Milk protein allergy: Nutritional and hygienic aspects. Ital. J. Pediatr. 2019, 45, 102. [Google Scholar] [CrossRef]
- Tesse, R.; Paglialunga, C.; Braccio, S.; Armenio, L. Adequacy and tolerance to ass’s milk in an Italian cohort of children with cow’s milk allergy. Ital. J. Pediatr. 2009, 35, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Barni, S.; Sarti, L.; Mori, F.; Muscas, G.; Belli, F.; Pucci, N.; Novembre, E. Tolerability and palatability of donkey’s milk in children with cow’s milk allergy. Pediatr. Allergy Immunol. 2018, 29, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Monti, G.; Bertino, E.; Muratore Cristina, M.; Coscia, A.; Cresi, F.; Silvestro, L.; Fabris, C.; Fortunato, D.; Giuffrida Gabriella, M.; Conti, A. Efficacy of donkey’s milk in treating highly problematic cow’s milk allergic children: An in vivo and in vitro study. Pediatr. Allergy Immunol. 2007, 18, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Monti, G.; Viola, S.; Baro, C.; Cresi, F.; Tovo, P.A.; Moro, G.; Ferrero, M.P.; Conti, A.; Bertino, E. Tolerability of donkey’s milk in 92 highly-problematic cow’s milk allergic children. J. Biol. Regul. Homeost. Agents 2012, 26, 75–82. [Google Scholar] [PubMed]
- Vita, D.; Passalacqua, G.; Di Pasquale, G.; Caminiti, L.; Crisafulli, G.; Rulli, I.; Pajno, G.B. Ass’s milk in children with atopic dermatitis and cow’s milk allergy: Crossover comparison with goat’s milk. Pediatr. Allergy Immunol. 2007, 18, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; Swiontek, K.; Antonicelli, L.; Garritani, M.S.; Bilò, M.B.; Mistrello, G.; Amato, S.; Revets, D.; Ollert, M.; Morisset, M.; et al. Lysozyme, a new allergen in donkey’s milk. Clin. Exp. Allergy 2018, 48, 1521–1523. [Google Scholar] [CrossRef] [PubMed]
- Peeters, C.; Herman, A.; Baeck, M. Donkey’s milk allergy. Br. J. Dermatol. 2017, 177, 1760–1761. [Google Scholar] [CrossRef]
- Giorgis, V.; Rolla, G.; Raie, A.; Geuna, M.; Boita, M.; Lamberti, C.; Nebbia, S.; Giribaldi, M.; Giuffrida, M.G.; Brussino, L.; et al. A case of work-related donkey milk allergy. J. Investig. Allergol. Clin. Immunol. 2018, 28, 197–199. [Google Scholar] [CrossRef]
- Uniacke-Lowe, T.; Huppertz, T.; Fox, P.F. Equine milk proteins: Chemistry, structure and nutritional significance. Int. Dairy J. 2010, 20, 609–629. [Google Scholar] [CrossRef]
- Doreau, M.; Martin-Rosset, W. Horse. In Animals that Produce Dairy Foods; Academic Press: Cambridge, MA, USA, 2011; pp. 358–364. [Google Scholar]
- Businco, L.; Giampietro, P.G.; Lucenti, P.; Lucaroni, F.; Pini, C.; Di Felice, G.; Lacovacci, P.; Curadi, C.; Orlandi, M. Allergenicity of mare’s milk in children with cow’s milk allergy. J. Allergy Clin. Immunol. 2000, 105, 1031–1034. [Google Scholar] [CrossRef]
- Fotschki, J.; Szyc, A.M.; Laparra, J.M.; Markiewicz, L.H.; Wróblewska, B. Immune-modulating properties of horse milk administered to mice sensitized to cow milk. J. Dairy Sci. 2016, 99, 9395–9404. [Google Scholar] [CrossRef] [Green Version]
- Duan, C.; Ma, L.; Cai, L.; Li, X.; Ma, F.; Chen, J.; Huo, G.; Li, D. Comparison of allergenicity among cow, goat, and horse milks using a murine model of atopy. Food Funct. 2021, 12, 5417–5428. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, L.; Kerre, S.; Goossens, A. The unsuspected power of mare’s milk. Contact Dermat. 2016, 74, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Fanta, C.; Ebner, C. Allergy to Mare’s milk. Allergy 1998, 53, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Gall, H.; Kalveram, M.; Sick, H. Allergy to the heat-labile proteins alpha-lactalbumin and beta-lactoglobulin in mare’s milk. J. Allergy Clin. Immunol. 1996, 97, 1304–1307. [Google Scholar] [CrossRef]
- Fjeldsgaard, B.E.; Paulsen, B.S. Comparison of IgE-binding antigens in horse dander and a mixture of horse hair and skin scrapings. Allergy 1993, 48, 535–541. [Google Scholar] [CrossRef]
- Heller, M.C.; Keoleian, G.A.; Willett, W.C. Toward a life cycle-based, diet-level framework for food environmental impact and nutritional quality assessment: A critical review. Environ. Sci. Technol. 2013, 47, 12632–12647. [Google Scholar] [CrossRef]
- Martini, D.; Tucci, M.; Bradfield, J.; Di Giorgio, A.; Marino, M.; Del Bo’, C.; Porrini, M.; Riso, P. Principles of sustainable healthy diets in worldwide dietary guidelines: Efforts so far and future perspectives. Nutrients 2021, 13, 1827. [Google Scholar] [CrossRef]
- Green, H.; Broun, P.; Cook, D.; Cooper, K.; Drewnowski, A.; Pollard, D.; Sweeney, G.; Roulin, A. Healthy and sustainable diets for future generations. J. Sci. Food Agric. 2018, 98, 3219–3224. [Google Scholar] [CrossRef] [Green Version]
- Craig, W.J.; Fresán, U. International analysis of the nutritional content and a review of health benefits of non-dairy plant-based beverages. Nutrients 2021, 13, 842. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for Special Dietary Needs: Non-dairy Plant-based Milk Substitutes and Fermented Dairy-type Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef]
- Bridges, M. Moo-ove over, cow’s milk: The rise of plant-based dairy alternatives. Pract. Gastroenterol. 2018, 42, 20–27. [Google Scholar]
- Fructuoso, I.; Romão, B.; Han, H.; Raposo, A.; Ariza-Montes, A.; Araya-Castillo, L.; Zandonadi, R.P. An overview on nutritional aspects of plant-based beverages used as substitutes for cow’s milk. Nutrients 2021, 13, 2650. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef] [PubMed]
- Ziarno, M.; Cichońska, P. Lactic Acid Bacteria-Fermentable Cereal- and Pseudocereal- Based Beverages. Microorganisms 2021, 9, 2532. [Google Scholar] [CrossRef] [PubMed]
- Merritt, R.J.; Fleet, S.E.; Fifi, A.; Jump, C.; Schwartz, S.; Sentongo, T.; Duro, D.; Rudolph, J.; Turner, J. North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Position Paper: Plant-based Milks. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 276–281. [Google Scholar] [CrossRef]
- Le Louer, B.; Lemale, J.; Garcette, K.; Orzechowski, C.; Chalvon, A.; Girardet, J.P.; Tounian, P. Severe nutritional deficiences in young infants with inappropriate plant milk consumption. Arch. Pediatr. 2014, 21, 483–488. [Google Scholar] [CrossRef]
- Vitoria Miñana, I. The nutritional limitations of plant-based beverages in infancy and childhood. Nutr. Hosp. 2017, 34, 1205–1214. [Google Scholar] [CrossRef] [Green Version]
- Agostoni, C.; Axelsson, I.; Goulet, O.; Koletzko, B.; Michaelsen, K.F.; Puntis, J.; Rieu, D.; Rigo, J.; Shamir, R.; Szajewska, H.; et al. Soy protein infant formulae and follow-on formulae: A commentary by the ESPGHAN committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Verduci, E.; Di Profio, E.; Cerrato, L.; Nuzzi, G.; Riva, L.; Vizzari, G.; D’Auria, E.; Giannì, M.L.; Zuccotti, G.; Peroni, D.G. Use of Soy-Based Formulas and Cow’s Milk Allergy: Lights and Shadows. Front. Pediatr. 2020, 8, 736. [Google Scholar] [CrossRef]
- Global Soy-Based Infant Formula Market Growth, Share, Size, Trends and Forecast (2021–2027). Report ID: Rn743009364. 2021. Available online: https://www.expertmarketresearch.com/reports/soy-based-infant-formula-market (accessed on 15 November 2021).
- Vandenplas, Y.; De Greef, E.; Devreker, T.; Hauser, B. Soy infant formula: Is it that bad? Acta Paediatr. Int. J. Paediatr. 2011, 100, 162–166. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Castrellon, P.G.; Rivas, R.; Gutiérrez, C.J.; Garcia, L.D.; Jimenez, J.E.; Anzo, A.; Hegar, B.; Alarcon, P. Safety of soya-based infant formulas in children. Br. J. Nutr. 2014, 111, 1340–1360. [Google Scholar] [CrossRef] [PubMed]
- Westmark, C.J. Soy-Based Therapeutic Baby Formulas: Testable Hypotheses Regarding the Pros and Cons. Front. Nutr. 2017, 3, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, Y.; Gutierrez-Castrellon, P.; González, M.G.; Rivas, R.; Lee, B.W.; Alarcon, P. A comprehensive review of sensitization and allergy to soy-based products. Clin. Rev. Allergy Immunol. 2014, 46, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Kattan, J.D.; Cocco, R.R.; Järvinen, K.M. Milk and Soy Allergy. Pediatr. Clin. N. Am. 2011, 58, 407–426. [Google Scholar] [CrossRef]
- Kerner, J. Use of Infant Formulas in Preventing or Postponing Atopic Manifestations. J. Pediatr. Gastroenterol. Nutr. 1997, 24, 442–446. [Google Scholar] [CrossRef]
- Rozenfeld, P.; Docena, G.H.; Añón, M.C.; Fossati, C.A. Detection and identification of a soy protein component that cross-reacts with caseins from cow’s milk. Clin. Exp. Immunol. 2002, 130, 49–58. [Google Scholar] [CrossRef]
- Giampietro, P.G.; Ragno, V.; Daniele, S.; Cantani, A.; Ferrara, M.; Businco, L. Soy hypersensitivity in children with food allergy. Ann. Allergy 1992, 69, 143–146. [Google Scholar]
- Bock, S.A.; Atkins, F.M. Patterns of food hypersensitivity during sixteen years of double-blind, placebo-controlled food challenges. J. Pediatr. 1990, 117, 561–567. [Google Scholar] [CrossRef]
- Klemola, T.; Vanto, T.; Juntunen-Backman, K.; Kalimo, K.; Korpela, R.; Varjonen, E. Allergy to soy formula and to extensively hydrolyzed whey formula in infants with cow’s milk allergy: A prospective, randomized study with a follow-up to the age of 2 years. J. Pediatr. 2002, 140, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Zeiger, R.S.; Sampson, H.A.; Bock, S.A.; Burks, A.W.; Harden, K.; Noone, S.; Martin, D.; Leung, S.; Wilson, G. Soy allergy in infants and children with IgE-associated cow’s milk allergy. J. Pediatr. 1999, 134, 614–622. [Google Scholar] [CrossRef]
- Johnstone, D.; Dutton, A. Dietary Prophylaxis of Allergic Disease in Children. N. Engl. J. Med. 1994, 274, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Glaster, J. Prophylaxis of Allergic Disease in the Newborn. J. Am. Med. Assoc. 1953, 153, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Kjellman, N.I.; Johansson, S.G. Soy versus cow’s milk in infants with a biparental history of atopic disease: Development of atopic disease and immunoglobulins from birth to 4 years of age. Clin. Allergy 1979, 9, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Gruskay, F.L. Comparison of Breast, Cow, and Soy Feedings in the Prevention of Onset of Allergic Disease: A 15-Year Prospective Study. Clin. Pediatr. 1982, 21, 486–491. [Google Scholar] [CrossRef]
- Osborn, D.A.; Sinn, J.K. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev. 2004, 3, CD003741. [Google Scholar]
- Vale, S.L.; Lobb, M.; Netting, M.J.; Murray, K.; Clifford, R.; Campbell, D.E.; Salter, S.M. A systematic review of infant feeding food allergy prevention guidelines—Can we AGREE? World Allergy Organ. J. 2021, 14, 100550. [Google Scholar] [CrossRef]
- Terracciano, L.; Bouygue, G.R.; Sarratud, T.; Veglia, F.; Martelli, A.; Fiocchi, A. Impact of dietary regimen on the duration of cow’s milk allergy: A random allocation study. Clin. Exp. Allergy 2010, 40, 637–642. [Google Scholar] [CrossRef]
- Berni Canani, R.; Nocerino, R.; Terrin, G.; Frediani, T.; Lucarelli, S.; Cosenza, L.; Passariello, A.; Leone, L.; Granata, V.; Di Costanzo, M.; et al. Formula selection for management of children with cow’s milk allergy influences the rate of acquisition of tolerance: A prospective multicenter study. J. Pediatr. 2013, 163, 771–777.e1. [Google Scholar] [CrossRef]
- Juliano, B.O.; Tuaño, A.P.P. Gross structure and composition of the rice grain. In Rice: Chemistry and Technology; Woodhead Publishing: Sawston, UK, 2018; pp. 31–53. [Google Scholar]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Composition and protein profile analysis of rice protein ingredients. J. Food Compos. Anal. 2017, 59, 18–26. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. The composition, extraction, functionality and applications of rice proteins: A review. Trends Food Sci. Technol. 2017, 64, 1–12. [Google Scholar] [CrossRef]
- Dupont, C.; Bocquet, A.; Tomé, D.; Bernard, M.; Campeotto, F.; Dumond, P.; Essex, A.; Frelut, M.L.; Guénard-Bilbault, L.; Lack, G.; et al. Hydrolyzed rice protein-based formulas, a vegetal alternative in cow’s milk allergy. Nutrients 2020, 12, 2654. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, A.; Dupont, C.; Chouraqui, J.P.; Darmaun, D.; Feillet, F.; Frelut, M.L.; Girardet, J.P.; Hankard, R.; Lapillonne, A.; Rozé, J.C.; et al. Efficacy and safety of hydrolyzed rice-protein formulas for the treatment of cow’s milk protein allergy. Arch. Pediatr. 2019, 26, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Communities TC of the E. Comission Directive 1999/21/EC of 25 March 1999 on Dietary Foods for Special Medical Purposes; pp. 1–11. 1999, 79. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1999L0021:20070119:EN:PDF (accessed on 15 January 2022).
- Regulation (EU) No. 609/2013 of the European Parlament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/. Off. J. Eur. Union. 2013. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX:32013R0609 (accessed on 15 January 2022).
- Agostoni, C.; Fiocchi, A.; Riva, E.; Terracciano, L.; Sarratud, T.; Martelli, A.; Lodi, F.; D’Auria, E.; Zuccotti, G.; Giovannini, M. Growth of infants with IgE-mediated cow’s milk allergy fed different formulas in the complementary feeding period. Pediatr. Allergy Immunol. 2007, 18, 599–606. [Google Scholar] [CrossRef]
- Vandenplas, Y.; De Greef, E.; Hauser, B. Safety and tolerance of a new extensively hydrolyzed rice protein-based formula in the management of infants with cow’s milk protein allergy. Eur. J. Pediatr. 2014, 173, 1209–1216. [Google Scholar] [CrossRef] [Green Version]
- Fiocchi, A.; Travaini, M.; D’Auria, E.; Banderali, G.; Bernardo, L.; Riva, E. Tolerance to a rice hydrolysate formula in children allergic to cow’s milk and soy. Clin. Exp. Allergy 2003, 33, 1576–1580. [Google Scholar] [CrossRef]
- Tzifi, F.; Grammeniatis, V.; Papadopoulos, M. Soy- and rice-based formula and infant allergic to cow’s milk. Endocr. Metab. Immune Disord.-Drug Targets 2014, 14, 38–46. [Google Scholar] [CrossRef]
- Fiocchi, A.; Restani, P.; Bernardini, R.; Lucarelli, S.; Lombardi, G.; Magazzù, G.; Marseglia, G.L.; Pittschieler, K.; Tripodi, S.; Troncone, R.; et al. A hydrolysed rice-based formula is tolerated by children with cow’s milk allergy: A multi-centre study. Clin. Exp. Allergy 2006, 36, 311–316. [Google Scholar] [CrossRef]
- Reche, M.; Pascual, C.; Fiandor, A.; Polanco, I.; Rivero-Urgell, M.; Chifre, R.; Johnston, S.; Martín-Esteban, M. The effect of a partially hydrolysed formula based on rice protein in the treatment of infants with cow’s milk protein allergy. Pediatr. Allergy Immunol. 2010, 21, 577–585. [Google Scholar] [CrossRef] [Green Version]
- Venlet, N.V.; Hettinga, K.A.; Schebesta, H.; Bernaz, N. Perspective: A Legal and Nutritional Perspective on the Introduction of Quinoa-Based Infant and Follow-on Formula in the EU. Adv. Nutr. 2021, 12, 1100–1107. [Google Scholar] [CrossRef]
- Le Roux, L.; Ménard, O.; Chacon, R.; Dupont, D.; Jeantet, R.; Deglaire, A.; Nau, F. Are faba bean and pea proteins potential whey protein substitutes in infant formulas? An in vitro dynamic digestion approach. Foods 2020, 9, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Roux, L.; Chacon, R.; Dupont, D.; Jeantet, R.; Deglaire, A.; Nau, F. In vitro static digestion reveals how plant proteins modulate model infant formula digestibility. Food Res. Int. 2020, 130, 108917. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, L.; Mejean, S.; Chacon, R.; Lopez, C.; Dupont, D.; Deglaire, A.; Nau, F.; Jeantet, R. Plant proteins partially replacing dairy proteins greatly influence infant formula functionalities. LWT 2020, 120, 108891. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; Barone, G.; Waldron, D.; Bez, J.; Joehnke, M.S.; Petersen, I.L.; Zannini, E.; Arendt, E.K.; O’Mahony, J.A. Formulation, pilot-scale preparation, physicochemical characterization and digestibility of a lentil protein-based model infant formula powder. J. Sci. Food Agric. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schuh, S.; Thevenier, A.; Ran-Ressler, R.; Rade-Kukic, K.; Kuslys, M. Infant Formula for Cow’s Milk Protein Allergic Infants. U.S. Patent Application 16/332,181, 29 August 2019. [Google Scholar]
- Ulloa, J.A.; Valencia, M.E.; Garcia, Z.H. Protein Concentrate from Chickpea: Nutritive Value of a Protein Concentrate from Chickpea (Cicer arietinum) Obtained by Ultrafiltration and Its Potential Use in an Infant Formula. J. Food Sci. 1988, 53, 1396–1398. [Google Scholar] [CrossRef]
- Malunga, L.N.; Bar-El Dadon, S.; Zinal, E.; Berkovich, Z.; Abbo, S.; Reifen, R. The potential use of chickpeas in development of infant follow-on formula. Nutr. J. 2014, 13, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; Naska, A.; et al. Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA J. 2016, 14, e04594. [Google Scholar]
- Floris, R.; Lambers, T.; Alting, A.; Kiers, J. Trends in infant formulas: A dairy perspective. In Improving the Safety and Quality of Milk: Improving Quality in Milk Products; Woodhead Publishing Limited: Sawston, UK, 2010; pp. 454–474. [Google Scholar] [CrossRef]
- Hildebrand, H.V.; Arias, A.; Simons, E.; Gerdts, J.; Povolo, B.; Rothney, J.; Protudjer, J.L.P. Adult and Pediatric Food Allergy to Chickpea, Pea, Lentil, and Lupine: A Scoping Review. J. Allergy Clin. Immunol. Pract. 2021, 9, 290–301. [Google Scholar] [CrossRef]
- Verma, A.K.; Kumar, S.; Das, M.; Dwivedi, P.D. A comprehensive review of legume allergy. Clin. Rev. Allergy Immunol. 2013, 45, 30–46. [Google Scholar] [CrossRef]
- Soller, L.; La Vieille, S.; Cameron, S.B.; Mak, R.; Cook, V.E.; Gerdts, J.; Chan, E.S. Allergic reactions to emerging food allergens in Canadian children. Allergy Asthma Clin. Immunol. 2021, 17, 71. [Google Scholar] [CrossRef]
- Damiani, E.; Aloia, A.M.; Priore, M.G.; Pastore, A.; Nardulli, S.; Lippolis, C.; Macchia, L.; Ferrannini, A. Vicia faba Hypersensitivity and ASA Intolerance in a Farmer: A Case Report. J. Allergy 2011, 2011, 191787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.; Kumar, S.; Verma, A.K.; Sharma, A.; Tripathi, A.; Chaudhari, B.P.; Kant, S.; Das, M.; Jain, S.K.; Dwivedi, P.D. Hypersensitivity linked to exposure of broad bean protein(s) in allergic patients and BALB/c mice. Nutrition 2014, 30, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Marsh, J.T.; Koppelman, S.J.; Kabourek, J.L.; Johnson, P.E.; Baumert, J.L. A perspective on pea allergy and pea allergens. Trends Food Sci. Technol. 2021, 116, 186–198. [Google Scholar] [CrossRef]
- Ruales, J.; de Grijalva, Y.; Lopez-Jaramillo, P.; Nair, B.M. The nutritional quality of an infant food from quinoa and its effect on the plasma level of insulin-like growth factor-1 (IGF-1) in undernourished children. Int. J. Food Sci. Nutr. 2002, 53, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Rojas, W.; Alandia, G.; Irigoyen, J.; Blajos, J.; Santivañez, T. Quinoa: An Ancient Crop to Contribute to World Food Security; FAO Regional Office for Latin America and the Caribbean: Santiago, Chile, 2011; Volume 37, p. 66. [Google Scholar]
- Ballegaard, A.S.R.; Larsen, J.M.; Rasmussen, P.H.; Untersmayr, E.; Pilegaard, K.; Bøgh, K.L. Quinoa (Chenopodium quinoa Willd.) Seeds Increase Intestinal Protein Uptake. Mol. Nutr. Food Res. 2021, 65, 2100102. [Google Scholar] [CrossRef]
- Astier, C.; Moneret-Vautrin, D.A.; Puillandre, E.; Bihain, B. First case report of anaphylaxis to quinoa, a novel food in France. Allergy 2008, 64, 818–819. [Google Scholar] [CrossRef]
- Guarnieri, G.; Bonadonna, P.; Olivieri, E.; Schiappoli, M. Occupational asthma induced by exposure to Quinoa. J. Investig. Allergol. Clin. Immunol. 2019, 29, 451–452. [Google Scholar] [CrossRef]
- Hong, J.; Convers, K.; Reeves, N.; Temparo, J. Anaphylaxis to quinoa. Ann. Allergy Asthma Immunol. 2013, 10, 60–61. [Google Scholar] [CrossRef]
- Ballegaard, A.S.R.; Sancho, A.I.; Knudsen, N.H.; Gupta, S.; Pilegaard, K.; Bøgh, K.L. Quinoa allergenicity—Identification of sensitising and cross-reactive quinoa proteins. Allergy Eur. J. Allergy Clin. Immunol. 2021, 76, 338–339. [Google Scholar]
- Kobayashi, T.; Nakamura, M.; Matsunaga, K.; Nakata, J.; Tagami, K.; Sato, N.; Kawabe, T.; Kondo, Y. Anaphylaxis due to potato starch (possibly caused by percutaneous sensitization). Asia Pac. Allergy 2021, 11, 5–9. [Google Scholar] [CrossRef]





| Cow’s Milk Fraction | Protein | Allergen Name | Content (%) | Size (kDa) | Major/Minor Allergen | S-S Bridges |
|---|---|---|---|---|---|---|
| Casein (80%) | αs1-casein | Bos d 9 | 32 | 23.6 | Major | 0 |
| αs2-casein | Bos d 10 | 10 | 25.2 | Major | 0 | |
| β-casein | Bos d 11 | 28 | 24 | Major | 0 | |
| ĸ-casein | Bos d 12 | 10 | 19 | Major | 1 | |
| Whey (20%) | α-lactalbumin | Bos d 4 | 5 | 14.2 | Major | 4 |
| β-lactoglobulin | Bos d 5 | 10 | 18.3 | Major | 2 + 1 free | |
| Serum albumin | Bos d 6 | 1 | 66.3 | Minor | 17 + 1 free | |
| Immunoglobulins | Bos d 7 | 3 | 160 | Minor | number varies 1 | |
| Lactoferrin | <1 | 80 | Minor | 16 |
| Protein | Goat | Sheep | Camel | Donkey 1 | Horse 1 | Human |
|---|---|---|---|---|---|---|
| αs1-casein | 91 | 91 | 67 | 57 | 55 | 33 |
| αs2-casein | 88 | 88 | 47 | 46 | 46 | NA |
| β-casein | 88 | 89 | 56 | 60 | 57 | 55 |
| ĸ-casein | 85 | 85 | 58 | 57 | 56 | 52 |
| α-lactalbumin | 95 | 95 | 60 | 47 | 60 | 74 |
| β-lactoglobulin | 93 | 93 | NA | 52 | 51 | NA |
| Serum albumin | 88 | 91 | 81 | 74 | 74 | 76 |
| Lactoferrin | 92 | 92 | 75 | 73 | 73 | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maryniak, N.Z.; Sancho, A.I.; Hansen, E.B.; Bøgh, K.L. Alternatives to Cow’s Milk-Based Infant Formulas in the Prevention and Management of Cow’s Milk Allergy. Foods 2022, 11, 926. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11070926
Maryniak NZ, Sancho AI, Hansen EB, Bøgh KL. Alternatives to Cow’s Milk-Based Infant Formulas in the Prevention and Management of Cow’s Milk Allergy. Foods. 2022; 11(7):926. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11070926
Chicago/Turabian StyleMaryniak, Natalia Zofia, Ana Isabel Sancho, Egon Bech Hansen, and Katrine Lindholm Bøgh. 2022. "Alternatives to Cow’s Milk-Based Infant Formulas in the Prevention and Management of Cow’s Milk Allergy" Foods 11, no. 7: 926. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11070926