Regulation Effects of Beeswax in the Intermediate Oil Phase on the Stability, Oral Sensation and Flavor Release Properties of Pickering Double Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
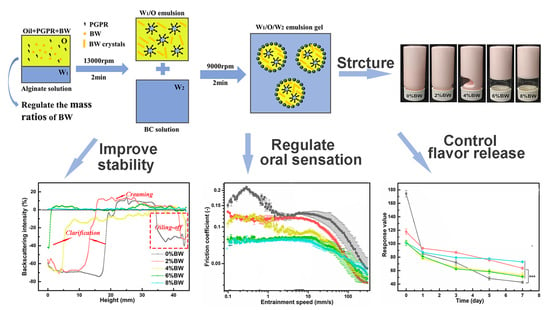
2.2. Preparation of Pickering Double Emulsions
2.3. Microstructure Observation
2.4. Rheological and Tribological Measurements
2.5. Determination of Stability during Storage and Processing
2.6. Flavor Release Properties of Pickering Double Emulsions
2.7. Statistical Analysis
3. Results and Discussion
3.1. Formation and Characterization of Double Emulsions
3.2. Effects of BW Addition on the Rheological and Tribological Properties of Double Emulsions
3.3. Effects of BW Addition on the Storage Stability of Double Emulsions
3.4. Effects of BW Addition on the Processing Stability of Double Emulsions
3.5. Effects of BW Addition on the Flavor Release Profile of Double Emulsions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muschiolik, G.; Dickinson, E. Double emulsions relevant to food systems: Preparation, stability, and applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 532–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Guo, B.; Deng, C.; Tang, C.; Liu, C.; Hu, X. Fabrication and characterization of the W/O/W multiple emulsion through oleogelation of oil. Food Chem. 2021, 358, 129856. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, M.; Zhao, Z.; Chen, X.; Cai, J.; Cao, Y.; Xiao, J. Lactobacillus acidophilus loaded Pickering double emulsion with enhanced viability and colon-adhesion efficiency. LWT—Food Sci. Technol. 2020, 121, 108928. [Google Scholar] [CrossRef]
- Chiu, N.; Tarrega, A.; Parmenter, C.; Hewson, L.; Wolf, B.; Fisk, I.D. Optimisation of octinyl succinic anhydride starch stablised W1/O/W2 emulsions for oral destablisation of encapsulated salt and enhanced saltiness. Food Hydrocoll. 2017, 69, 450–458. [Google Scholar] [CrossRef]
- Aditya, N.P.; Aditya, S.; Yang, H.; Kim, H.W.; Park, S.O.; Ko, S. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef]
- Muschiolik, G. Multiple emulsions for food use. Curr. Opin. Colloid Interface Sci. 2007, 12, 213–220. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; Yin, J.; Chang, F.; Wang, C.; He, X.; Huang, Q.; Zhang, B. Anthocyanin-loaded double Pickering emulsion stabilized by octenylsuccinate quinoa starch: Preparation, stability and in vitro gastrointestinal digestion. Int. J. Biol. Macromol. 2020, 152, 1233–1241. [Google Scholar] [CrossRef]
- Stasse, M.; Laurichesse, E.; Vandroux, M.; Ribaut, T.; Héroguez, V.; Schmitt, V. Cross-linking of double oil-in-water-in-oil emulsions: A new way for fragrance encapsulation with tunable sustained release. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125448. [Google Scholar] [CrossRef]
- Ma, L.; Wan, Z.; Yang, X. Multiple water-in-oil-in-water emulsion gels based on self-assembled saponin fibrillar network for photosensitive cargo protection. J. Agric. Food Chem. 2017, 65, 9735–9743. [Google Scholar] [CrossRef]
- Oppermann, A.K.L.; Renssen, M.; Schuch, A.; Stieger, M.; Scholten, E. Effect of gelation of inner dispersed phase on stability of (W1/O/W2) multiple emulsions. Food Hydrocoll. 2015, 48, 17–26. [Google Scholar] [CrossRef]
- Iqbal, S.; Chen, X.D.; Kirk, T.V.; Huang, H. Controlling the rheological properties of W1/O/W2 multiple emulsions using osmotic swelling: Impact of WPI-pectin gelation in the internal and external aqueous phases. Colloids Surf. B Biointerfaces 2020, 185, 110629. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Xia, Q. In vitro digestion behavior of (W1/O/W2) double emulsions incorporated in alginate hydrogel beads: Microstructure, lipolysis, and release. Food Hydrocoll. 2020, 107, 105950. [Google Scholar] [CrossRef]
- Singh, A.; Auzanneau, F.I.; Rogers, M.A. Advances in edible oleogel technologies—A decade in review. Food Res. Int. 2017, 97, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, M.; Xia, Q. Improved stability of (W1/O/W2) double emulsions based on dual gelation: Oleogels and hydrogels. J. Food Process Eng. 2019, 42, e13186. [Google Scholar] [CrossRef]
- Spyropoulos, F.; Frasch-Melnik, S.; Norton, I.T. W/O/W emulsions stabilized by fat crystals—Their formulation, stability and ability to retain salt. Procedia Food Sci. 2011, 1, 1700–1708. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-W.; Guo, J.; Wang, J.-M.; Yin, S.-W.; Yang, X.-Q. Controlled volatile release of structured emulsions based on phytosterols crystallization. Food Hydrocoll. 2016, 56, 170–179. [Google Scholar] [CrossRef]
- Sun, R.; Song, G.; Zhang, H.; Zhang, H.; Chi, Y.; Ma, Y.; Li, H.; Bai, S.; Zhang, X. Effect of basil essential oil and beeswax incorporation on the physical, structural, and antibacterial properties of chitosan emulsion based coating for eggs preservation. LWT—Food Sci. Technol. 2021, 150, 112020. [Google Scholar] [CrossRef]
- Chaireh, S.; Ngasatool, P.; Kaewtatip, K. Novel composite foam made from starch and water hyacinth with beeswax coating for food packaging applications. Int. J. Biol. Macromol. 2020, 165, 1382–1391. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Zhang, R.; Gao, Y.; Mao, L. Novel high internal phase emulsions with gelled oil phase: Preparation, characterization and stability evaluation. Food Hydrocoll. 2021, 121, 106995. [Google Scholar] [CrossRef]
- Anvari, M.; Joyner, H.S. Effect of formulation on structure-function relationships of concentrated emulsions: Rheological, tribological, and microstructural characterization. Food Hydrocoll. 2017, 72, 11–26. [Google Scholar] [CrossRef]
- Oppermann, A.K.L. Influence of double (W1/O/W2) emulsion composition on lubrication properties. Food Funct. 2017, 8, 522–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Tu, Z.; Zou, Z.; Shangguan, X.; Wang, H.; Bansal, N. Glycosylated fish gelatin emulsion: Rheological, tribological properties and its application as model coffee creamers. Food Hydrocoll. 2020, 102, 105552. [Google Scholar] [CrossRef]
- Matos, M.; Gutiérrez, G.; Martínez-Rey, L.; Iglesias, O.; Pazos, C. Encapsulation of resveratrol using food-grade concentrated double emulsions: Emulsion characterization and rheological behaviour. J. Food Eng. 2018, 226, 73–81. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, L.; Yan, W.; Gao, C.; Jia, X. Preparation and characterization of a novel soy protein isolate-sugar beet pectin emulsion gel and its application as a multi-phased nutrient carrier. Foods. 2022, 11, 469. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.R.; Boehm, M.W.; Baier, S.K. Oral processing, texture and mouthfeel: From rheology to tribology and beyond. Curr. Opin. Colloid Interface Sci. 2013, 18, 349–359. [Google Scholar] [CrossRef] [Green Version]
- De Lavergne, M.D.; van de Velde, F.; Stieger, M. Bolus matters: The influence of food oral breakdown on dynamic texture perception. Food Funct. 2017, 8, 464–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, R.; Chen, J. Smoothness as a tactile percept: Correlating ‘oral’ tribology with sensory measurements. Food Hydrocoll. 2019, 87, 38–47. [Google Scholar] [CrossRef]
- Chen, J.; Stokes, J.R. Rheology and tribology: Two distinctive regimes of food texture sensation. Trends Food Sci. Technol. 2012, 25, 4–12. [Google Scholar] [CrossRef]
- Nguyen, P.T.M.; Kravchuk, O.; Bhandari, B.; Prakash, S. Effect of different hydrocolloids on texture, rheology, tribology and sensory perception of texture and mouthfeel of low-fat pot-set yoghurt. Food Hydrocoll. 2017, 72, 90–104. [Google Scholar] [CrossRef] [Green Version]
- Laiho, S.; Williams, R.P.W.; Poelman, A.; Appelqvist, I.; Logan, A. Effect of whey protein phase volume on the tribology, rheology and sensory properties of fat-free stirred yoghurts. Food Hydrocoll. 2017, 67, 166–177. [Google Scholar] [CrossRef]
- Olivares, M.L.; Costabel, L.M.; Zorrilla, S.E.; De Vicente, J. Calcium-induced skim milk gels: Effect of milk powder concentration and pH on tribo-rheological characteristics and gel physico-chemical properties. Food Hydrocoll. 2022, 124, 107335. [Google Scholar] [CrossRef]
- Jin, W.; Pan, Y.; Wu, Y.; Chen, C.; Xu, W.; Peng, D.; Huang, Q. Structural and interfacial characterization of oil bodies extracted from Camellia oleifera under the neutral and alkaline condition. LWT—Food Sci. Technol. 2021, 141, 110911. [Google Scholar] [CrossRef]
- Sanhueza, L.; García, P.; Giménez, B.; Benito, J.M.; Matos, M.; Gutiérrez, G. Encapsulation of pomegranate peel extract (Punica granatum L.) by double emulsions: Effect of the encapsulation method and oil phase. Foods 2022, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Qi, X.; Ren, T.; Huang, Y.; Keller, A.A.; Wang, H.; Wu, B.; Jin, H.; Li, F. Heteroaggregation of CeO2 and TiO2 engineered nanoparticles in the aqueous phase: Application of turbiscan stability index and fluorescence excitation-emission matrix (EEM) spectra. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Zhang, J.; Cao, Y.; Wang, J.; Xiao, J. Influence of microcrystalline cellulose on the microrheological property and freeze-thaw stability of soybean protein hydrolysate stabilized curcumin emulsion. LWT—Food Sci. Technol. 2016, 66, 590–597. [Google Scholar] [CrossRef]
- Diaz-Ruiz, R.; Valdeon, I.; Alvarez, J.R.; Matos, M.; Gutierrez, G. Simultaneous encapsulation of trans-resveratrol and vitamin D3 in highly concentrated double emulsions. J. Sci. Food Agric. 2021, 101, 3654–3664. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Zhang, N.; Lin, W.F.; Tang, C.H. Freeze-thaw stability of Pickering emulsions stabilized by soy and whey protein particles. Food Hydrocoll. 2017, 69, 173–184. [Google Scholar] [CrossRef]
- Liu, J.; Kharat, M.; Tan, Y.; Zhou, H.; Mundo, J.L.M.; Mcclements, D.J. Impact of fat crystallization on the resistance of W/O/W emulsions to osmotic stress: Potential for temperature-triggered release. Food Res. Int. 2020, 134, 109273. [Google Scholar] [CrossRef]
- Mao, L.; Roos, Y.H.; Miao, S. Study on the rheological properties and volatile release of cold-set emulsion-filled protein gels. J. Agric. Food Chem. 2014, 62, 11420–11428. [Google Scholar] [CrossRef]
- Mao, L.; Miao, S.; Yuan, F.; Gao, Y. Study on the textural and volatile characteristics of emulsion filled protein gels as influenced by different fat substitutes. Food Res. Int. 2018, 103, 1–7. [Google Scholar] [CrossRef]
- Mao, L.; O’kennedy, B.T.; Roos, Y.H.; Hannon, J.A.; Miao, S. Effect of monoglyceride self-assembled structure on emulsion properties and subsequent flavor release. Food Res. Int. 2012, 48, 233–240. [Google Scholar] [CrossRef]







| Group | End of LVR | Crossover Point | ||||
|---|---|---|---|---|---|---|
| Τ (pa) | G′ (pa) | G″ (pa) | Γ (%) | Τ (pa) | G′ = G″ (pa) | |
| 4.0% BW | 2.12 ± 0.03 | 159.80 ± 9.72 | 158.70 ± 1.71 | 0.10 ± 0.00 | 0.18 ± 0.01 | 125.50 ± 3.43 |
| 6.0% BW | 2.34 ± 0.08 | 519.90 ± 16.94 | 241.40 ± 6.74 | 1.38 ± 0.06 | 4.30 ± 0.15 | 221.40 ± 1.80 |
| 8.0% BW | 4.12 ± 0.19 | 758.40 ± 7.27 | 304.90 ± 1.10 | 7.98 ± 0.36 | 15.22 ± 0.05 | 135.50 ± 5.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Abdullah; Wang, W.; Xiao, J. Regulation Effects of Beeswax in the Intermediate Oil Phase on the Stability, Oral Sensation and Flavor Release Properties of Pickering Double Emulsions. Foods 2022, 11, 1039. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11071039
Chen M, Abdullah, Wang W, Xiao J. Regulation Effects of Beeswax in the Intermediate Oil Phase on the Stability, Oral Sensation and Flavor Release Properties of Pickering Double Emulsions. Foods. 2022; 11(7):1039. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11071039
Chicago/Turabian StyleChen, Meimiao, Abdullah, Wenbo Wang, and Jie Xiao. 2022. "Regulation Effects of Beeswax in the Intermediate Oil Phase on the Stability, Oral Sensation and Flavor Release Properties of Pickering Double Emulsions" Foods 11, no. 7: 1039. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11071039