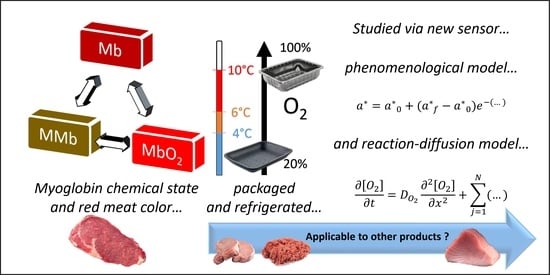
Predicting the Oxidative Degradation of Raw Beef Meat during Cold Storage Using Numerical Simulations and Sensors—Prospects for Meat and Fish Foods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Meat Products
2.2. Sensor to Track the Time-Course of Oxidation
2.3. Color Measurements and Comparison with ORP
2.4. Development of the Mathematical Models
2.4.1. Phenomenological Model
2.4.2. Reaction–Diffusion Model
3. Results
3.1. Identification of Parameters S and Ea in the Reaction–Diffusion Model
3.2. Effect of Temperature and Oxygen Content on the Kinetics of a* Calculated by the Models
4. Discussion and Prospects
4.1. Prospects for the Modeling Approaches
4.2. Prospects for Sensors
4.3. Extension to Other Meats and Fish
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Symbol | Significance | Unit |
|---|---|---|
| a*, a*0, a*f | Redness coordinate in the CIELAB system, at initial time or at final measurement time | |
| aij | Stoichiometric coefficient for compound i in reaction j | |
| Angle | Cutting direction relative to muscle fiber orientation | ° |
| b* | Yellowness coordinate in the CIELAB system | |
| b0-b5 | Constant parameters of lag | |
| [Ci] | Concentration of compound i | |
| c0-c5 | ||
| Diffusivity of oxygen in meat at T | ||
| Ea | Activation energy for all the reactions | |
| kjT, kj20°C | Rate constants of reaction j at T or 20 °C | |
| L* | Lightness coordinate in the CIELAB system | |
| LL | Longissimus lumborum | |
| LT | Longissimus thoracis (core muscle of the ribeye) | |
| lag | Lag time parameter | |
| M | Number of compounds in the chemical system | |
| MAP | Modified-atmosphere packaging | |
| Mat | Aging time (set at 14 days in this study) | days |
| Mb | Deoxymyoglobin | |
| MbFe4+ | Ferrylmyoglobin | |
| MbO2 | Oxymyoglobin | |
| MMb | Metmyoglobin | |
| N | Number of reactions in the chemical system | |
| NADH | Nicotinamide adenine dinucleotide hydride | |
| Maximum evolution rate of a* | ||
| ORP | Oxidation–reduction potential | |
| pO2 | Oxygen fraction (in air or MAP) | % |
| R | Molar gas constant (=8.314) | |
| S | Threshold that separates the change in L* from the change in a* | |
| T | Temperature | °C, K |
| t | Time | s, days |
| x | Distance from the surface | m |
References
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision: ESA Working Paper No. 12-03; FAO Agricultural Economics Working Paper; FAO: Rome, Italy, 2012. [Google Scholar]
- Adesogan, A.T.; Havelaar, A.H.; McKune, S.L.; Eilittä, M.; Dahl, G.E. Animal source foods: Sustainability problem or malnutrition and sustainability solution? Perspective matters. Glob. Food Secur. 2019, 25, 100325. [Google Scholar] [CrossRef]
- Wang, Z.; Tu, J.; Zhou, H.; Lu, A.; Xu, B. A comprehensive insight into the effects of microbial spoilage, myoglobin autoxidation, lipid oxidation, and protein oxidation on the discoloration of rabbit meat during retail display. Meat Sci. 2021, 172, 108359. [Google Scholar] [CrossRef] [PubMed]
- Fernández–López, J.; Pérez–Alvarez, J.A.; Aranda–Catalá, V. Effect of mincing degree on colour properties in pork meat. Color Res. Appl. 2000, 25, 376–380. [Google Scholar] [CrossRef]
- Ha, M.; Warner, R.D.; King, C.; Wu, S.; Ponnampalam, E.N. Retail Packaging Affects Colour, Water Holding Capacity, Texture and Oxidation of Sheep Meat more than Breed and Finishing Feed. Foods 2022, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Domínguez, R.; Lorenzo, J.M.; Bohrer, B.M. The Relationship between Lipid Content in Ground Beef Patties with Rate of Discoloration and Lipid Oxidation during Simulated Retail Display. Foods 2021, 10, 1982. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Richards, M.P.; Undeland, I. Lipid oxidation and antioxidant delivery systems in muscle food. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1275–1299. [Google Scholar] [CrossRef]
- Singh, A.; Mittal, A.; Benjakul, S. Undesirable discoloration in edible fish muscle: Impact of indigenous pigments, chemical reactions, processing, and its prevention. Compr. Rev. Food Sci. Food Saf. 2021, 21, 580–603. [Google Scholar] [CrossRef]
- Buchanan, J.G.; Thomas, P.M. Improving the Color Shelf Life of Farmed Southern Bluefin Tuna (Thunnus maccoyii)Flesh with Dietary Supplements of Vitamins E and C and Selenium. J. Aquat. Food Prod. Technol. 2008, 17, 285–302. [Google Scholar] [CrossRef]
- Ponnampalam, E.; Butler, K.; Muir, S.; Plozza, T.; Kerr, M.; Brown, W.; Jacobs, J.; Knight, M. Lipid Oxidation and Colour Stability of Lamb and Yearling Meat (Muscle Longissimus Lumborum) from Sheep Supplemented with Camelina-Based Diets after Short-, Medium-, and Long-Term Storage. Antioxidants 2021, 10, 166. [Google Scholar] [CrossRef]
- Gatellier, P.; Mercier, Y.; Juin, H.; Renerre, M. Effect of finishing mode (pasture- or mixed-diet) on lipid composition, colour stability and lipid oxidation in meat from Charolais cattle. Meat Sci. 2005, 69, 175–186. [Google Scholar] [CrossRef]
- Descalzo, A.; Sancho, A. A review of natural antioxidants and their effects on oxidative status, odor and quality of fresh beef produced in Argentina. Meat Sci. 2008, 79, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M. Critical overview of the use of plant antioxidants in the meat industry: Opportunities, innovative applications and future perspectives. Meat Sci. 2021, 181, 108610. [Google Scholar] [CrossRef] [PubMed]
- Jongberg, S.; Skov, S.H.; Tørngren, M.A.; Skibsted, L.H.; Lund, M.N. Effect of white grape extract and modified atmosphere packaging on lipid and protein oxidation in chill stored beef patties. Food Chem. 2011, 128, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Chatli, M.; Sahoo, J. Antioxidant potential of curry (Murraya koenigii L.) and mint (Mentha spicata) leaf extracts and their effect on colour and oxidative stability of raw ground pork meat during refrigeration storage. Food Chem. 2012, 133, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Xiang, R.; Tang, D.; Zhu, M.; Liu, X. Regulation of protein oxidation in Cantonese sausages by rutin, quercetin and caffeic acid. Meat Sci. 2020, 175, 108422. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Domínguez, R.; Lorenzo, J. Recent Research Advances in Meat Products. Foods 2021, 10, 1303. [Google Scholar] [CrossRef]
- Jurčaga, L.; Bobko, M.; Kolesárová, A.; Bobková, A.; Demianová, A.; Haščík, P.; Belej, L.; Mendelová, A.; Bučko, O.; Kročko, M.; et al. Blackcurrant (Ribes nigrum L.) and Kamchatka Honeysuckle (Lonicera caerulea var. Kamtschatica) Extract Effects on Technological Properties, Sensory Quality, and Lipid Oxidation of Raw-Cooked Meat Product (Frankfurters). Foods 2021, 10, 2957. [Google Scholar] [CrossRef]
- Zhou, B.; Luo, J.; Quan, W.; Lou, A.; Shen, Q. Antioxidant Activity and Sensory Quality of Bacon. Foods 2022, 11, 236. [Google Scholar] [CrossRef]
- Gutiérrez-Del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, A.; Tuñón-Granda, M.; Miguélez, E.; Villar, C.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Rathod, N.B.; Ranveer, R.C.; Benjakul, S.; Kim, S.; Pagarkar, A.U.; Patange, S.; Ozogul, F. Recent developments of natural antimicrobials and antioxidants on fish and fishery food products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4182–4210. [Google Scholar] [CrossRef]
- López-Fernández, O.; Bohrer, B.M.; Munekata, P.E.; Domínguez, R.; Pateiro, M.; Lorenzo, J.M. Improving oxidative stability of foods with apple-derived polyphenols. Compr. Rev. Food Sci. Food Saf. 2021, 21, 296–320. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; López, E.M.S.; Rodríguez, J.A.; Barros, L.; Lorenzo, J.M. Potential Use of Elderberry (Sambucus nigra L.) as Natural Colorant and Antioxidant in the Food Industry. A Review. Foods 2021, 10, 2713. [Google Scholar] [CrossRef] [PubMed]
- Giménez, B.; Gómez-Guillén, M.; Pérez-Mateos, M.; Montero, P.; Márquez-Ruiz, G. Evaluation of lipid oxidation in horse mackerel patties covered with borage-containing film during frozen storage. Food Chem. 2011, 124, 1393–1403. [Google Scholar] [CrossRef] [Green Version]
- Vital, A.P.; Guerrero, A.; Guarnido, P.; Severino, I.C.; Olleta, J.; Blasco, M.; Prado, I.N.D.; Maggi, F.; Campo, M. Effect of Active-Edible Coating and Essential Oils on Lamb Patties Oxidation during Display. Foods 2021, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Smaoui, S.; Ben Hlima, H.; Tavares, L.; Ennouri, K.; Ben Braiek, O.; Mellouli, L.; Abdelkafi, S.; Khaneghah, A.M. Application of essential oils in meat packaging: A systemic review of recent literature. Food Control 2021, 132, 108566. [Google Scholar] [CrossRef]
- Tanaka, R.; Nakazawa, N.; Maeda, T.; Fukushima, H.; Wada, R.; Sugiura, Y.; Matsushita, T.; Hatate, H.; Fukuda, Y. Effects of Chilled Storage, Freezing Rates, and Frozen Storage Temperature on Lipid Oxidation in Meat Blocks from Cultured Bluefin Tuna Thunnus thynnus. J. Aquat. Food Prod. Technol. 2016, 25, 1073–1085. [Google Scholar] [CrossRef]
- Corlett, M.; Pethick, D.; Kelman, K.; Jacob, R.; Gardner, G. Consumer Perceptions of Meat Redness Were Strongly Influenced by Storage and Display Times. Foods 2021, 10, 540. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Bohrer, B.; Lorenzo, J.M. Protein Oxidation in Muscle Foods: A Comprehensive Review. Antioxidants 2021, 11, 60. [Google Scholar] [CrossRef]
- Tao, Y.; Ma, L.; Li, D.; Tian, Y.; Liu, J.; Liu, D. Proteomics analysis to investigate the effect of oxidized protein on meat color and water holding capacity in Tan mutton under low temperature storage. LWT-Food Sci. Technol. 2021, 146, 111429. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Guo, A. Animal and Plant Protein Oxidation: Chemical and Functional Property Significance. Foods 2020, 10, 40. [Google Scholar] [CrossRef]
- Warner, R.; Kearney, G.; Hopkins, D.; Jacob, R. Retail colour stability of lamb meat is influenced by breed type, muscle, packaging and iron concentration. Meat Sci. 2017, 129, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Guider, J.; Skovgaar, I.; Staun, H.; Skibsted, L.; Jensen, S.; Møller, A.; Buckley, J.; Bertelsen, G. Effects of dietary α-tocopheryl acetate supplementation on α-tocopherol deposition in porcine m. psoas major and m. longissimus dorsi and on drip loss, colour stability and oxidative stability of pork meat. Meat Sci. 1997, 45, 491–500. [Google Scholar] [CrossRef]
- Sáenz, C.; Hernández, B.; Alberdi, C.; Alfonso, S.; Diñeiro, J.M. A multispectral imaging technique to determine concentration profiles of myoglobin derivatives during meat oxygenation. Eur. Food Res. Technol. 2008, 227, 1329–1338. [Google Scholar] [CrossRef]
- Tofteskov, J.; Hansen, J.; Bailey, N. Modelling the autoxidation of myoglobin in fresh meat under modified atmosphere packing conditions. J. Food Eng. 2017, 214, 129–136. [Google Scholar] [CrossRef]
- Kondjoyan, A.; Sicard, J.; Badaroux, M.; Gatellier, P. Kinetics analysis of the reactions responsible for myoglobin chemical state in meat using an advanced reaction–diffusion model. Meat Sci. under review.
- Ramanathan, R.; Hunt, M.C.; Mancini, R.A.; Nair, M.N.; Denzer, M.L.; Suman, S.P.; Mafi, G.G. Recent Updates in Meat Color Research: Integrating Traditional and High-Throughput Approaches. Meat Muscle Biol. 2020, 4, 1–24. [Google Scholar] [CrossRef]
- Cattlemen’s Beef Board and National Cattlemen’s Beef Association. The Guide to Identifying Meat Cuts. Available online: https://agrilife.org/4hmeat/files/2018/01/Guide-To-ID-Meat-Cuts.pdf (accessed on 2 March 2022).
- Sheridan, J.J.; Allen, P.; Ziegler, J.H.; Marinkov, M.; Suvakov, M.D.; Heinz, G. Guidelines for Slaughtering, Meat Cutting and Further Processing. Available online: https://www.fao.org/3/T0279E/T0279E00.htm (accessed on 2 March 2022).
- Kauffman, R.G.; Habel, R.E.; Smulders, F.J.M.; Hartman, W.; Bergström, P.L. Recommended terminology for the muscle commonly designated ‘longissimus dorsi’. Meat Sci. 1990, 28, 259–265. [Google Scholar] [CrossRef]
- Cucci, P.; N’Gatta, A.C.K.; Sanguansuk, S.; Lebert, A.; Audonnet, F. Relationship between Color and Redox Potential (Eh) in Beef Meat Juice. Validation on Beef Meat. Appl. Sci. 2020, 10, 3164–3177. [Google Scholar] [CrossRef]
- Cucci, P. Development of a New Method of Measurement, Modeling and Prediction of Redox Potential (pOR) in Preparation for Rational Improvements of Meat Products Transformation Processes (Développement d’une Nouvelle Méthode de Mesure, Modélisation et Prédiction du Potentiel D’oxydoréduction (pOR) en Vue D’une Amélioration Raisonnée des Procédés de Trans-Formation des Produits Carnés). Ph.D. Thesis, University Clermont Auvergne, Clermont-Ferrand, France, 2020. [Google Scholar]
- Holman, B.W.; Hopkins, D.L. The use of conventional laboratory-based methods to predict consumer acceptance of beef and sheep meat: A review. Meat Sci. 2021, 181, 108586. [Google Scholar] [CrossRef]
- Seyfert, M.; Mancini, R.A.; Hunt, M.C. Internal premature browning in cooked ground beef patties from high-oxygen modi-fied-atmosphere packaging. J. Food Sci. 2004, 69, C721–C725. [Google Scholar] [CrossRef]
- Chaix, E.; Guillaume, C.; Guillard, V. Oxygen and Carbon Dioxide Solubility and Diffusivity in Solid Food Matrices: A Review of Past and Current Knowledge. Compr. Rev. Food Sci. Food Saf. 2014, 13, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Limbo, S.; Sinelli, N.; Torri, L.; Riva, M. Freshness decay and shelf life predictive modelling of European sea bass (Dicentrarchus labrax) applying chemical methods and electronic nose. LWT-Food Sci. Tech. 2009, 42, 977–984. [Google Scholar] [CrossRef]




| S | 2 | 2 | 3 | 3 | 2 | |
| Ea (kJ/mol) | 30 | 35 | 30 | 35 | 32.5 | |
| Mesh | 100 | 100 | 100 | 100 | 400 | |
| O2 | T (°C) | Roots of the residual sum of squares | ||||
| 20% | 2 | 0.37 | 0.34 | 0.39 | 0.42 | 0.09 |
| 6 | 0.27 | 0.24 | 0.30 | 0.33 | 0.20 | |
| 10 | 0.39 | 0.36 | 0.43 | 0.46 | 0.27 | |
| 100% | 2 | 0.17 | 0.26 | 0.15 | 0.13 | 0.20 |
| 6 | 0.13 | 0.11 | 0.17 | 0.22 | 0.11 | |
| 10 | 0.16 | 0.13 | 0.21 | 0.25 | 0.15 | |
| Mean | 0.25 | 0.24 | 0.28 | 0.30 | 0.17 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondjoyan, A.; Sicard, J.; Cucci, P.; Audonnet, F.; Elhayel, H.; Lebert, A.; Scislowski, V. Predicting the Oxidative Degradation of Raw Beef Meat during Cold Storage Using Numerical Simulations and Sensors—Prospects for Meat and Fish Foods. Foods 2022, 11, 1139. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11081139
Kondjoyan A, Sicard J, Cucci P, Audonnet F, Elhayel H, Lebert A, Scislowski V. Predicting the Oxidative Degradation of Raw Beef Meat during Cold Storage Using Numerical Simulations and Sensors—Prospects for Meat and Fish Foods. Foods. 2022; 11(8):1139. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11081139
Chicago/Turabian StyleKondjoyan, Alain, Jason Sicard, Paolo Cucci, Fabrice Audonnet, Hiba Elhayel, André Lebert, and Valérie Scislowski. 2022. "Predicting the Oxidative Degradation of Raw Beef Meat during Cold Storage Using Numerical Simulations and Sensors—Prospects for Meat and Fish Foods" Foods 11, no. 8: 1139. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11081139