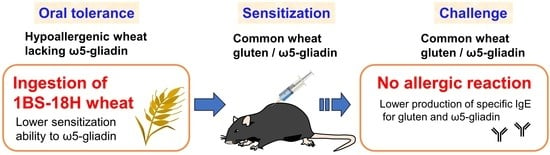
Hypoallergenic Wheat Line (1BS-18H) Lacking ω5-Gliadin Induces Oral Tolerance to Wheat Gluten Proteins in a Rat Model of Wheat Allergy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Western Blot Analysis against Wheat Gluten
2.3. Animals
2.4. Induction of OT to Gluten and Its Components in Rats
2.5. Measurement of Plasma Levels of IgE and IgG1 Abs Specific for Gluten and Its Components
2.6. Evaluation of Systemic Anaphylaxis
2.7. Statistical Analyses
3. Results
3.1. Western Blot Analysis against Wheat Gluten
3.2. Induction of OT to Gluten in Rats
3.3. Effect of 1BS-18H Gluten on the Induction of OT to Normal Gluten
3.4. Effect of 1BS-18H Gluten on the Induction of OT to ω5-Gliadin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ebisawa, M.; Ito, K.; Fujisawa, T.; Committee for Japanese Pediatric Guideline for Food Allergy; The Japanese Society of Pediatric Allergy and Clinical Immunology; Japanese Society of Allergology. Japanese guidelines for food allergy 2020. Allergol. Int. 2020, 69, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Ogino, R.; Chinuki, Y.; Yokooji, T.; Takizawa, D.; Matsuo, H.; Morita, E. Identification of peroxidase-1 and beta-glucosidase as cross-reactive wheat allergens in grass pollen-related wheat allergy. Allergol. Int. 2021, 70, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Morita, E.; Matsuo, H.; Chinuki, Y.; Takahashi, H.; Dahlström, J.; Tanaka, A. Food-dependent exercise-induced anaphylaxis -importance of omega-5 gliadin and HMW-glutenin as causative antigens for wheat-dependent exercise-induced anaphylaxis-. Allergol. Int. 2009, 58, 493–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aihara, Y.; Takahashi, Y.; Kotoyori, T.; Mitsuda, T.; Ito, R.; Aihara, M.; Ikezawa, Z.; Yokota, S. Frequency of food-dependent, exercise-induced anaphylaxis in Japanese junior-high-school students. J. Allergy Clin. Immunol. 2001, 108, 1035–1039. [Google Scholar] [CrossRef]
- Scherf, K.A.; Brockow, K.; Biedermann, T.; Koehler, P.; Wieser, H. Wheat-dependent exercise-induced anaphylaxis. Clin. Exp. Allergy. 2016, 46, 10–20. [Google Scholar] [CrossRef]
- Matsuo, H.; Yokooji, T.; Taogoshi, T. Common food allergens and their IgE-binding epitopes. Allergol. Int. 2015, 64, 332–343. [Google Scholar] [CrossRef] [Green Version]
- Yokooji, T.; Kurihara, S.; Murakami, T.; Chinuki, Y.; Takahashi, H.; Morita, E.; Harada, S.; Ishii, K.; Hiragun, M.; Hide, M.; et al. Characterization of causative allergens for wheat-dependent exercise-induced anaphylaxis sensitized with hydrolyzed wheat proteins in facial soap. Allergol. Int. 2013, 62, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Cummings, A.J.; Knibb, R.C.; King, R.M.; Lucas, J.S. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: A review. Allergy 2010, 65, 933–945. [Google Scholar] [CrossRef] [Green Version]
- Herman, E.M.; Helm, R.M.; Jung, R.; Kinney, A.J. Genetic modification removes an immunodominant allergen from soybean. Plant Physiol. 2003, 132, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Kim, J.H.; Yook, H.S.; Kang, K.O.; Lee, S.Y.; Hwang, H.J.; Byun, M.W. Effects of gamma radiation on the allergenic and antigenic properties of milk proteins. J. Food Prot. 2001, 64, 272–276. [Google Scholar] [CrossRef]
- Altenbach, S.B.; Tanaka, C.K.; Pineau, F.; Lupi, R.; Drouet, M.; Beaudouin, E.; Morisset, M.; Denery-Papini, S. Assessment of the Allergenic Potential of Transgenic Wheat (Triticum aestivum) with Reduced Levels of ω5-Gliadins, the Major Sensitizing Allergen in Wheat-Dependent Exercise-Induced Anaphylaxis. J. Agric. Food Chem. 2015, 63, 9323–9332. [Google Scholar] [CrossRef]
- Altenbach, S.B.; Chang, H.C.; Simon-Buss, A.; Jang, Y.R.; Denery-Papini, S.; Pineau, F.; Gu, Y.Q.; Huo, N.; Lim, S.H.; Kang, C.S.; et al. Towards reducing the immunogenic potential of wheat flour: Omega gliadins encoded by the D genome of hexaploid wheat may also harbor epitopes for the serious food allergy WDEIA. BMC Plant Biol. 2018, 18, 291. [Google Scholar] [CrossRef]
- Altenbach, S.B.; Allen, P.V. Transformation of the US bread wheat ‘Butte 86’ and silencing of omega-5 gliadin genes. GM Crops 2011, 2, 66–73. [Google Scholar] [CrossRef]
- Altenbach, S.B.; Tanaka, C.K.; Seabourn, B.W. Silencing of omega-5 gliadins in transgenic wheat eliminates a major source of environmental variability and improves dough mixing properties of flour. BMC Plant Biol. 2014, 14, 393. [Google Scholar] [CrossRef] [Green Version]
- Komoto, K.; Okamoto, S.; Hamada, M.; Obana, N.; Samori, M.; Imamura, T. Japanese Consumer Perceptions of Genetically Modified Food: Findings from an International Comparative Study. Interact. J. Med. Res. 2016, 5, e23. [Google Scholar] [CrossRef] [Green Version]
- Kohno, K.; Takahashi, H.; Endo, T.R.; Matsuo, H.; Shiwaku, K.; Morita, E. Characterization of a hypoallergenic wheat line lacking ω-5 gliadin. Allergol. Int. 2016, 65, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Wu, D.; Guo, L.; Yao, Y.; Yao, X.; Wang, Z.; Wu, K.; Cao, X.; Gao, X. Effects of Quinoa Flour on Wheat Dough Quality, Baking Quality, and in vitro Starch Digestibility of the Crispy Biscuits. Front. Nutr. 2022, 9, 846808. [Google Scholar] [CrossRef]
- Yokooji, T.; Nouma, H.; Ogino, R.; Taogoshi, T.; Morita, E.; Matsuo, H. Quantification of the ω5- and γ-gliadin content in wheat flour and rat plasma with an enzyme-linked immunosorbent assay using antibodies specific to their IgE-binding epitopes. Allergol. Int. 2019, 68, 112–113. [Google Scholar] [CrossRef]
- Yamada, Y.; Yokooji, T.; Ninomiya, N.; Taogoshi, T.; Morita, E.; Matsuo, H. Evaluation of the allergenicity of ω5-gliadin-deficient Hokushin wheat (1BS-18) in a wheat allergy rat model. Biochem. Biophys. Rep. 2019, 20, 100702. [Google Scholar] [CrossRef]
- Lack, G. Epidemiologic risks for food allergy. J. Allergy Clin. Immunol. 2008, 121, 1331–1336. [Google Scholar] [CrossRef]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. LEAP Study Team. Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Perkin, M.R.; Logan, K.; Tseng, A.; Raji, B.; Ayis, S.; Peacock, J.; Brough, H.; Marrs, T.; Radulovic, S.; Craven, J.; et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N. Engl. J. Med. 2016, 374, 1733–1743. [Google Scholar] [CrossRef] [Green Version]
- Wieser, H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. [Google Scholar] [CrossRef]
- Matsuo, H.; Kohno, K.; Morita, E. Molecular cloning, recombinant expression and IgE-binding epitope of omega-5 gliadin, a major allergen in wheat-dependent exercise-induced anaphylaxis. FEBS J. 2005, 272, 4431–4438. [Google Scholar] [CrossRef]
- Kumagai, H.; Suda, A.; Sakurai, H.; Kumagai, H.; Arai, S.; Inomata, N.; Ikezawa, Z. Improvement of digestibility, reduction in allergenicity, and induction of oral tolerance of wheat gliadin by deamidation. Biosci. Biotechnol. Biochem. 2007, 71, 977–985. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, T.; Yokooji, T.; Hirano, T.; Kataoka, Y.; Taogoshi, T.; Matsuo, H. Aspirin enhances sensitization to the egg-white allergen ovalbumin in rats. PLoS ONE 2019, 14, e0226165. [Google Scholar] [CrossRef] [Green Version]
- Yokooji, T.; Matsuo, H. Sodium Cromoglycate Prevents Exacerbation of IgE-mediated Food-Allergic Reaction Induced by Aspirin in a Rat Model of Egg Allergy. Int. Arch. Allergy Immunol. 2015, 167, 193–202. [Google Scholar] [CrossRef]
- Cassidy, B.G.; Dvorak, J.; Anderson, O.D. The wheat low-molecular-weight glutenin genes: Characterization of six new genes and progress in understanding gene family structure. Theor. Appl. Genet. 1998, 96, 743–750. [Google Scholar] [CrossRef]
- Schiavi, E.; Smolinska, S.; O’Mahony, L. Intestinal dendritic cells. Curr. Opin. Gastroenterol. 2015, 31, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Bertolini, T.B.; Biswas, M.; Terhorst, C.; Daniell, H.; Herzog, R.W.; Piñeros, A.R. Role of orally induced regulatory T cells in immunotherapy and tolerance. Cell Immunol. 2021, 359, 104251. [Google Scholar] [CrossRef]
- Chen, Y.; Inobe, J.; Marks, R.; Gonnella, P.; Kuchroo, V.K.; Weiner, H.L. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature 1995, 376, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Battais, F.; Mothes, T.; Moneret-Vautrin, D.A.; Pineau, F.; Kanny, G.; Popineau, Y.; Bodinier, M.; Denery-Papini, S. Identification of IgE-binding epitopes on gliadins for patients with food allergy to wheat. Allergy 2005, 60, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Morita, E.; Tatham, A.S.; Morimoto, K.; Horikawa, T.; Osuna, H.; Ikezawa, Z.; Kaneko, S.; Kohno, K.; Dekio, S. Identification of the IgE-binding epitope in omega-5 gliadin, a major allergen in wheat-dependent exercise-induced anaphylaxis. J. Biol. Chem. 2004, 279, 12135–12140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuta, T.; Tanaka, K.; Tagami, K.; Matsui, T.; Sugiura, S.; Kando, N.; Kanie, Y.; Naito, M.; Izumi, H.; Tanaka, A.; et al. Exercise-induced allergic reactions on desensitization to wheat after rush oral immunotherapy. Allergy 2020, 75, 1414–1422. [Google Scholar] [CrossRef]
- Watanabe, M.; Suzuki, T.; Ikezawa, Z.; Arai, S. Controlled enzymatic treatment of wheat proteins for production of hypoallergenic flour. Biosci. Biotechnol. Biochem. 1994, 58, 388–390. [Google Scholar] [CrossRef]
- Li, X.; Miyakawa, T.; Takano, T.; Nakajima-Adachi, H.; Tanokura, M.; Hachimura, S. Induction of Oral Tolerance by Pepsin-Digested Gliadin Retaining T Cell Reactivity in a Mouse Model of Wheat Allergy. Int. Arch. Allergy Immunol. 2020, 181, 446–455. [Google Scholar] [CrossRef]
- Abe, R.; Shimizu, S.; Yasuda, K.; Sugai, M.; Okada, Y.; Chiba, K.; Akao, M.; Kumagai, H.; Kumagai, H. Evaluation of reduced allergenicity of deamidated gliadin in a mouse model of wheat-gliadin allergy using an antibody prepared by a peptide containing three epitopes. J. Agric. Food Chem. 2014, 62, 2845–2852. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, Y.; Yokooji, T.; Kunimoto, K.; Inoguchi, K.; Ogino, R.; Taogoshi, T.; Morita, E.; Matsuo, H. Hypoallergenic Wheat Line (1BS-18H) Lacking ω5-Gliadin Induces Oral Tolerance to Wheat Gluten Proteins in a Rat Model of Wheat Allergy. Foods 2022, 11, 2181. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11152181
Yamada Y, Yokooji T, Kunimoto K, Inoguchi K, Ogino R, Taogoshi T, Morita E, Matsuo H. Hypoallergenic Wheat Line (1BS-18H) Lacking ω5-Gliadin Induces Oral Tolerance to Wheat Gluten Proteins in a Rat Model of Wheat Allergy. Foods. 2022; 11(15):2181. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11152181
Chicago/Turabian StyleYamada, Yukinori, Tomoharu Yokooji, Kyohei Kunimoto, Koki Inoguchi, Ryohei Ogino, Takanori Taogoshi, Eishin Morita, and Hiroaki Matsuo. 2022. "Hypoallergenic Wheat Line (1BS-18H) Lacking ω5-Gliadin Induces Oral Tolerance to Wheat Gluten Proteins in a Rat Model of Wheat Allergy" Foods 11, no. 15: 2181. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11152181