Effects of Bifidobacteria Fermentation on Physico-Chemical, Thermal and Structural Properties of Wheat Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Wheat Starch Separated from Fermented Dough
2.3. Determination of Damaged Starch Content
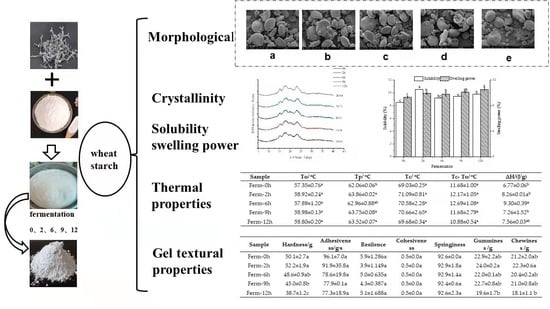
2.4. Morphological Properties
2.5. Solubility and Swelling Power
2.6. X-ray Diffraction (XRD)
2.7. Thermal Properties
2.8. Pasting Properties
2.9. Gel Textural Properties
2.10. Statistical Analysis
3. Results and Discussion
3.1. Damaged Starch Content during Bifidobacteria Fermentation
3.2. Morphological Property
3.3. Solubility and Swelling Power
3.4. Changes in Crystallinity during Fermentation
3.5. Thermal Properties
3.6. Pasting Properties
3.7. Gel Textural Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahaman, S.; Singh, A.; Praveen, S.; Krishnan, V. Low digestible starch and food industry: A changing paradigm. Indian J. Exp. Biol. 2020, 58, 830–841. [Google Scholar]
- Wang, Q.; Li, L.; Zheng, X. Recent advances in heat-moisture modified cereal starch: Structure, functionality and its applications in starchy food systems. Food Chem. 2020, 344, 128700. [Google Scholar]
- Zhao, T.; Li, X.; Zhu, R.; Ma, Z.; Liu, L.; Wang, X.; Hu, X. Effect of natural fermentation on the structure and physicochemical properties of wheat starch. Carbohydr. Polym. 2019, 218, 163–169. [Google Scholar]
- Tabara, A.; Miyajima, C.; Moki, N.; Kasahara, F.; Seguchi, M. Improvement of Bread Making Properties by the Addition of Alginates. Food Sci. Technol. Res. 2016, 22, 145–151. [Google Scholar]
- Galle, S.; Schwab, C.; Arendt, E.; Gänzle, M. Exopolysaccharide-Forming Weissella Strains as Starter Cultures for Sorghum and Wheat Sourdoughs. J. Agric. Food Chem. 2010, 58, 5834–5841. [Google Scholar]
- Galle, S.; Arendt, E.K. Exopolysaccharides from Sourdough Lactic Acid Bacteria. Crit. Rev. Food Sci. Nutr. 2014, 54, 891–901. [Google Scholar]
- Falade, A.T.; Emmambux, M.N.; Buys, E.M.; Taylor, J. Improvement of maize bread quality through modification of dough rheological properties by lactic acid bacteria fermentation. J. Cereal Sci. 2014, 60, 471–476. [Google Scholar]
- Gobbetti, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar]
- Vuyst, L.D.; Kerrebroeck, S.V.; Harth, H.; Huys, G.; Daniel, H.M.; Weckx, S. Microbial ecology of sourdough fermentations: Diverse or uniform? Food Microbiol. 2014, 37, 11–29. [Google Scholar]
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar]
- Yang, Y.; Tao, W.Y. Effects of lactic acid fermentation on FT-IR and pasting properties of rice flour. Food Res. Int. 2008, 41, 937–940. [Google Scholar]
- Ma, S.; Wang, Z.; Guo, X.; Wang, F.; Wang, X. Sourdough improves the quality of whole-wheat flour products: Mechanisms and challenges—A review. Food Chem. 2021, 360, 130038. [Google Scholar]
- Reyes, I.; Cruz-Sosa, F.; Roman-Guerrero, A.; Alvarez-Ramirez, J.; Vernon-Carter, J. Structural changes of corn starch during Saccharomyces cerevisiae fermentation. Starch Staerke 2016, 68, 961–971. [Google Scholar]
- Lee, J.S.; Chung, M.J.; Seo, J.G. In Vitro Evaluation of Antimicrobial Activity of Lactic Acid Bacteria against Clostridium difficile. Toxicol. Res. 2013, 29, 99–106. [Google Scholar]
- Canquil, N.; Villarroel, M.; Bravo, S.; Rubilar, M.; Shene, C. Behavior of the rheological parameters of exopolysaccharides synthesized by three lactic acid bacteria. Carbohydr. Polym. 2007, 68, 270–279. [Google Scholar]
- Elsanhoty, R.M.; Ghonamy, A.G.; El-Adly, N.A. Impact of lactic acid bacteria and bifidobacterium on the survival of bacillus subtilus during fermentation of wheat sourdough. J. Food Process. Preserv. 2017, 41, e13086. [Google Scholar]
- Chavan, R.S.; Chavan, S.R. Sourdough technology-a traditional way for wholesome foods: A review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 169–182. [Google Scholar]
- Collado, M.C.; González, A.; González, R.; Hernández, M.; Ferrús, M.; Sanz, Y. Antimicrobial peptides are among the antagonistic metabolites produced by Bifidobacterium against Helicobacter pylori. Int. J. Antimicrob. Agents 2005, 25, 385–391. [Google Scholar]
- Sharma, M.; Wasan, A.; Sharma, R.K. Recent developments in probiotics: An emphasis on Bifidobacterium. Food Biosci. 2021, 41, 100993. [Google Scholar]
- Palacios, M.C.; Haros, M.; Sanz, Y.; Rosell, C.M. Phytate degradation by Bifidobacterium on whole wheat fermentation. Eur. Food Res. Technol. 2008, 226, 825–831. [Google Scholar]
- Kaplan, H.; Hutkins, R.W. Fermentation of Fructooligosaccharides by Lactic Acid Bacteria and Bifidobacteria. Appl. Environ. Microbiol. 2019, 66, 2682–2684. [Google Scholar]
- Liu, C.; Hong, J.; Zheng, X. Effect of heat-moisture treatment on morphological, structural and functional characteristics of ball-milled wheat starches. Starch Strke. 2017, 69, 1500141. [Google Scholar]
- Struyf, N.; Laurent, J.; Lefevere, B.; Verspreet, J.; Courtin, C.M. Establishing the relative importance of damaged starch and fructan as sources of fermentable sugars in wheat flour and whole meal bread dough fermentations. Food Chem. 2017, 218, 89–98. [Google Scholar]
- Hong, J.; Li, L.; Li, C.; Liu, C.; Bian, K. Effect of Heat–Moisture Treatment on Physicochemical, Thermal, Morphological, and Structural Properties of Mechanically Activated Large A- and Small B-Wheat Starch Granules. J. Food Sci. 2019, 84, 2795–2804. [Google Scholar]
- Ao, Z.; Jane, J.L. Characterization and modeling of the A- and B-granule starches of wheat, triticale, and barley. Carbohydr. Polym. 2007, 67, 46–55. [Google Scholar]
- Liu, S.; Ren, F.; Zhao, L.; Jiang, L.; Hao, Y.; Jin, J.; Zhang, M.; Guo, H.; Lei, X.; Sun, E. Starch and starch hydrolysates are favorable carbon sources for Bifidobacteria in the human gut. BMC Microbiol. 2015, 15, 54–63. [Google Scholar]
- Majzoobi, M.; Beparva, P. Effects of acetic acid and lactic acid on physicochemical characteristics of native and cross-linked wheat starches. Food Chem. 2014, 147, 312–317. [Google Scholar]
- Chinsamran, K.; Piyachomkwan, K.; Santisopasri, V.; Sriroth, K. Effect of Lactic Acid Fermentation on Physico-chemical Properties of Starch Derived from Cassava, Sweet Potato and Rice. Nat. Sci. 2005, 39, 76–87. [Google Scholar]
- Salmenkallio-Marttila, M.; Katina, K.; Autio, K. Effects of Bran Fermentation on Quality and Microstructure of High-Fiber Wheat Bread. Cereal Chem. 2001, 78, 429–435. [Google Scholar]
- Edema, M.O.; Emmambux, M.N.; Taylor, J.R.N. Improvement of fonio dough properties through starch modification by sourdough fermentation. Starch Staerke 2013, 65, 730–737. [Google Scholar]
- Zavareze, E.; Dias, A. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar]
- Sanz-Penella, J.M.; Tamayo-Ramos, J.A.; Haros, M. Application of Bifidobacteria as Starter Culture in Whole Wheat Sourdough Breadmaking. Food Bioprocess Technol. 2012, 5, 2370–2380. [Google Scholar]
- Gunaratne, A.; Corke, H. Influence of prior acid treatment on acetylation of wheat, potato and maize starches. Food Chem. 2007, 105, 917–925. [Google Scholar]
- Al-Ansi, W.; Sajid, B.; Mahdi, A.A.; Al-Maqtari, Q.A.; Wang, L. Molecular structure, morphological, and physicochemical properties of highlands barley starch as affected by natural fermentation. Food Chem. 2021, 356, 129665. [Google Scholar]
- Ye, F.; Xiao, L.; Liang, Y.; Zhou, Y.; Zhao, G. Spontaneous fermentation tunes the physicochemical properties of sweet potato starch by modifying the structure of starch molecules. Carbohydr. Polym. 2019, 213, 79–88. [Google Scholar]
- Deng, F.M.; Mu, T.H.; Zhang, M.; Abegunde, O.K. Composition, structure, and physicochemical properties of sweet potato starches isolated by sour liquid processing and centrifugation. Starch Staerke 2013, 65, 162–171. [Google Scholar]
- Olanipekun, B.F.; Otunola, E.T.; Adelakun, O.E.; Oyela, O.J. Effect of fermentation with Rhizopus oligosporus on some physico-chemical properties of starch extracts from soybean flour. Food Chem. Toxicol. 2009, 47, 1401–1405. [Google Scholar]
- Yulianto, A.; Widiyanti, P.T.; Suparman; Musa. Fermented Starch: Production Testing of Process Stabilization. Food Sci. Nutr. Stud. 2019, 3. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Yu, J.; Yu, J. Conformation and location of amorphous and semi-crystalline regions in C-type starch granules revealed by SEM, NMR and XRD. Food Chem. 2008, 110, 39–46. [Google Scholar]
- Zhang, B.; Li, X.; Jia, L.; Xie, F.; Ling, C. Supramolecular structure of A- and B-type granules of wheat starch. Food Hydrocoll. 2013, 31, 68–73. [Google Scholar]
- lata-Oviedo, M.; Camargo, C. Effect of acid treatments and drying processes on physico-chemical and functional properties of cassava starch. J. Sci. Food Agric. 1998, 77, 103–108. [Google Scholar]
- Hoover, R. Acid-Treated starches. Food Rev. Int. 2000, 16, 369–392. [Google Scholar]
- Utrilla-Coello, R.G.; Hernández-Jaimes, C.; Carrillo-Navas, H.; González, F.; Rodríguez, E.; Bello-Pérez, L.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Acid hydrolysis of native corn starch: Morphology, crystallinity, rheological and thermal properties. Carbohydr. Polym. 2014, 103, 596–602. [Google Scholar]
- Almeida, R.; Pereira, T.; Freire, V.; Santiago, Â.M.; Gusmão, R. Influence of enzymatic hydrolysis on the properties of red rice starch. Int. J. Biol. Macromol. 2019, 141, 1210–1219. [Google Scholar]
- Alonso-Gomez, L.; Nio-López, A.M.; Romero-Garzón, A.M.; Pineda-Gomez, P. Physicochemical transformation of cassava starch during fermentation for production of sour starch in Colombia. Starch Staerke 2016, 68, 1139–1147. [Google Scholar]
- Mestres, C.; Rouau, X.; Zakhia, N.; Brabet, C. Physicochemical Properties of Cassava Sour Starch; Ciat Publication: Turin, Italy, 1996. [Google Scholar]
- Idowu, M.A.; Adeyemi, I.A.; David, M. Sensory evaluation and nutrient composition of weaning food from pregelatinized maize-sweet potato mixtures. Plant Foods Hum. Nutr. 1993, 44, 149–155. [Google Scholar]
- Li, M.; Dhital, S.; Wei, Y. Multilevel Structure of Wheat Starch and Its Relationship to Noodle Eating Qualities. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1042–1055. [Google Scholar]
- Putri, W.D.R.; Haryadi; Marseno, D.W.; Cahyanto, M.N. Role of Lactic Acid Bacteria on Structural and Physicochemical Properties of Sour Cassava Starch. APCBEE Proc. 2012, 2, 104–109. [Google Scholar]
- Moorthy, S.N.; Mathew, G. Cassava fermentation and associated changes in physicochemical and functional properties. CRC Crit. Rev. Food Technol. 1998, 38, 73–121. [Google Scholar]
- Phothiset, S.; Charoenrein, S. Morphology and physicochemical changes in rice flour during rice paper production. Food Res. Int. 2007, 40, 266–272. [Google Scholar]
- Varavinit, S.; Shobsngob, S.; Varanyanond, W.; Chinachoti, P.; Naivikul, O. Effect of Amylose Content on Gelatinization, Retrogradation and Pasting Properties of Flours from Different Cultivars of Thai Rice. Starch Staerke 2003, 55, 410–415. [Google Scholar]
- Numfor, F.A.; Walter, W.M.; Schwartz, S.J. Emulsifiers affect the texture of pastes made from fermented and non-fermented cassava flours. Int. J. Food Sci. Technol. 1998, 33, 455–460. [Google Scholar]




| Sample | To/°C | Tp/°C | Tc/°C | Tc-To/°C | ∆H/(J/g) |
|---|---|---|---|---|---|
| Ferm-0 h | 57.35 ± 0.76 a | 62.06 ± 0.06 b | 69.03 ± 0.25 a | 11.68 ± 1.00 a | 6.77 ± 0.06 b |
| Ferm-2 h | 58.92 ± 0.24 a | 63.86 ± 0.02 a | 71.09 ± 0.81 a | 12.17 ± 1.05 a | 8.26 ± 0.01a b |
| Ferm-6 h | 57.89 ± 1.20 a | 62.96 ± 0.88 ab | 70.58 ± 2.28 a | 12.69 ± 1.08 a | 9.30 ± 0.39 a |
| Ferm-9 h | 58.98 ± 0.13 a | 63.75 ± 0.08 a | 70.66 ± 2.65 a | 11.68 ± 2.79 a | 7.26 ± 1.52 b |
| Ferm-12 h | 58.80 ± 0.20 a | 63.52 ± 0.07 a | 69.68 ± 0.34 a | 10.88 ± 0.54 a | 7.56 ± 0.03 ab |
| Sample | Peak Viscosity/cp | Trough/cP | Breakdown/cP | Final Viscosity/cP | Setback/cP |
|---|---|---|---|---|---|
| Ferm-0 h | 2247 ± 12.72 a | 1769.5 ± 27.58 a | 477.5 ± 40.30 a | 2816.5 ± 2.12 a | 1047 ± 29.69 a |
| Ferm-2 h | 2033 ± 32.53 b | 1512.5 ± 13.43 b | 520.5 ± 45.96 a | 2221 ± 14.14 c | 708.5 ± 0.71 d |
| Ferm-6 h | 2242.5 ± 28.99 a | 1735.5 ± 120.92 a | 507 ± 149.91 a | 2539.5 ± 101.12 b | 804 ± 19.79 c |
| Ferm-9 h | 2221 ± 66.47 a | 1653 ± 31.11 ab | 568 ± 35.35 a | 2530 ± 41.01 b | 877 ± 9.89 b |
| Ferm-12 h | 2168.5 ± 10.61 a | 1679.5 ± 45.96 a | 489 ± 35.35 a | 2413.5 ± 33.23 b | 734 ± 12.73 d |
| Sample | Hardness/g | Adhesiveness/g·s | Resilience | Cohesiveness | Springiness | Gumminess/g | Chewiness/g |
|---|---|---|---|---|---|---|---|
| Ferm-0 h | 50.1 ± 2.7 a | 96.1 ± 7.0 a | 5.9 ± 1.286 a | 0.5 ± 0.0 a | 92.6 ± 0.0 a | 22.9 ± 2.2 ab | 21.2 ± 2.0 ab |
| Ferm-2 h | 52.2 ± 1.9 a | 91.9 ± 35.8 a | 3.9 ± 1.149 a | 0.5 ± 0.0 a | 92.9 ± 1.8 a | 24.0 ± 0.2 a | 22.3 ± 0.6 a |
| Ferm-6 h | 48.6 ± 0.9 ab | 78.6 ± 19.8 a | 5.0 ± 0.635 a | 0.5 ± 0.0 a | 92.9 ± 1.4 a | 22.0 ± 0.1 ab | 20.4 ± 0.2 ab |
| Ferm-9 h | 45.0 ± 0.8 b | 77.9 ± 0.1 a | 4.3 ± 0.387 a | 0.5 ± 0.0 a | 92.4 ± 0.6 a | 22.7 ± 0.8 ab | 21.0 ± 0.8 ab |
| Ferm-12 h | 38.7 ± 1.2 c | 77.3 ± 18.9 a | 5.1 ± 1.688 a | 0.5 ± 0.0 a | 92.6 ± 2.3 a | 19.6 ± 1.7 b | 18.1 ± 1.1 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Guo, W.; Chen, P.; Liu, C.; Wei, J.; Zheng, X.; Saeed Omer, S.H. Effects of Bifidobacteria Fermentation on Physico-Chemical, Thermal and Structural Properties of Wheat Starch. Foods 2022, 11, 2585. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11172585
Hong J, Guo W, Chen P, Liu C, Wei J, Zheng X, Saeed Omer SH. Effects of Bifidobacteria Fermentation on Physico-Chemical, Thermal and Structural Properties of Wheat Starch. Foods. 2022; 11(17):2585. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11172585
Chicago/Turabian StyleHong, Jing, Wanxue Guo, Peixia Chen, Chong Liu, Juan Wei, Xueling Zheng, and Saeed Hamid Saeed Omer. 2022. "Effects of Bifidobacteria Fermentation on Physico-Chemical, Thermal and Structural Properties of Wheat Starch" Foods 11, no. 17: 2585. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11172585