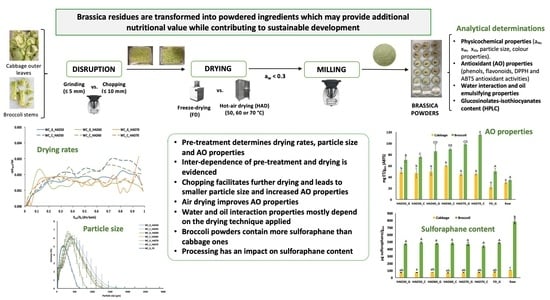
Impact of Disruption and Drying Conditions on Physicochemical, Functional and Antioxidant Properties of Powdered Ingredients Obtained from Brassica Vegetable By-Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Powder Manufacturing
2.2. Matrix Behaviour during Air Drying: Drying and Drying Rate Curves
2.3. Analytical Determinations
2.3.1. Physicochemical Properties
2.3.2. Water Interaction and Oil Emulsifying Properties
2.3.3. Antioxidant Properties
2.3.4. Glucosinolate and Isothiocyanate Content by High Pressure Liquid Chromatography (HPLC)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Drying and Drying Rate Curves
3.2. Physicochemical Characterization
3.3. Antioxidant Properties
3.4. Glucosinolate and Isothiocyanate Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burel, C.; Boujar, T.; Escaffre, A.M.; Kaushik, S.J.; Boeuf, G.; Mol, K.A.; Van der Geyten, S.; Kühn, E.R. Dietary low-glucosinolate rapeseed meal affects thyroid status and nutrient utilization in rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 2000, 83, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahuja, I.; Rohloff, J.; Bones, M. Defence mechanisms of Brassicaceae: Implications for plant-insect interactions and potential for integrated pest management. A review. Agron. Sustain. Dev. 2010, 2, 311–348. [Google Scholar] [CrossRef] [Green Version]
- Bohinc, T.; Ban, S.G.; Ban, D.; Trdan, S. Glucosinolates in plant protection strategies: A review. Arch. Biol. Sci. 2012, 64, 821–828. [Google Scholar] [CrossRef]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto, M.A.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar] [PubMed]
- Bischoff, K.L. Glucosinolates. Nutraceuticals Effic. Saf. Toxic. 2016, 40, 551–554. [Google Scholar]
- Zukalová, H.; Vašák, J. The role and effects of glucosinolates of Brassica species—A review. Plant Soil Environ. 2002, 48, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Kuljarachanan, T.; Chiewchan, N.; Devahastin, S. Profiles of major glucosinolates in different parts of white cabbage and their evolutions during processing into vegetable powder by various methods. Int. Food Res. J. 2019, 26, 1763–1772. [Google Scholar]
- Textor, S.; Gershenzon, J. Herbivore induction of the glucosinolate-myrosinase defense system: Major trends, biochemical bases and ecological significance. Phytochem. Rev. 2009, 8, 149–170. [Google Scholar] [CrossRef] [Green Version]
- Angelino, D.; Jeffery, E. Glucosinolate hydrolysis and bioavailability of resulting isothiocyanates: Focus on glucoraphanin. J. Funct. Foods 2014, 7, 67–76. [Google Scholar] [CrossRef]
- Wagner, A.E.; Terschluesen, A.M.; Rimbach, G. Health promoting effects of brassica-derived phytochemicals: From chemopreventive and anti-inflammatory activities to epigenetic regulation. Oxid. Med. Cell. Longev. 2013, 2013, 964539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Kim, B.; Kim, S.H.; Srivastava, S.K. Molecular targets of isothiocyanates in cancer: Recent advances. Mol. Nutr. Food Res. 2014, 58, 1685–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joensuu, K.; Hartikainen, H.; Karppinen, S.; Jaakkonen, A.K.; Kuoppa-aho, M. Developing the collection of statistical food waste data on the primary production of fruit and vegetables. Environ. Sci. Pollut. Res. 2021, 28, 24618–24627. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 4 October 2022).
- FAO and the Sustainable Development Goals|FAO|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/about/strategy-programme-budget/strategic-framework/fao-sdg/en/ (accessed on 1 August 2022).
- Ferreira, M.S.L.; Santos, M.C.P.; Moro, T.M.A.; Basto, G.J.; Andrade, R.M.S.; Gonçalves, E.C.B.A. Formulation and characterization of functional foods based on fruit and vegetable residue flour. J. Food Sci. Technol. 2015, 52, 822–830. [Google Scholar] [CrossRef] [Green Version]
- Neacsu, M.; Vaughan, N.; Raikos, V.; Multari, S.; Duncan, G.J.; Duthie, G.G.; Russell, W.R. Phytochemical profile of commercially available food plant powders: Their potential role in healthier food reformulations. Food Chem. 2015, 179, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Badr, A.; Desjardins, Y.; Gosselin, A.; Angers, P. Characterization of industrial broccoli discards (Brassica oleracea var. italica) for their glucosinolate, polyphenol and flavonoid contents using UPLC MS/MS and spectrophotometric methods. Food Chem. 2018, 245, 1204–1211. [Google Scholar] [CrossRef]
- Mokhtar, S.M.; Swailam, H.M.; Embaby, H.E.S. Physicochemical properties, nutritional value and techno-functional properties of goldenberry (Physalis peruviana) waste powder concise title: Composition of goldenberry juice waste. Food Chem. 2018, 248, 1–7. [Google Scholar] [CrossRef]
- Karam, M.C.; Petit, J.; Zimmer, D.; Baudelaire Djantou, E.; Scher, J. Effects of drying and grinding in production of fruit and vegetable powders: A review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Yu, J.; Vallad, G.E.; Boyd, N.S. Evaluation of allyl isothiocyanate as a soil fumigant for tomato (Lycopersicon esculentum Mill.) production. Plant Dis. 2019, 103, 2764–2770. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Fan, L. Effects of different drying methods on the storage stability of barley grass powder. J. Sci. Food Agric. 2022, 102, 1076–1084. [Google Scholar] [CrossRef]
- Dovene, A.K.; Wang, L.; Bokhary, S.U.F.; Madebo, M.P.; Zheng, Y.; Jin, P. Effect of Cutting Styles on Quality and Antioxidant Activity of Stored Fresh-Cut Sweet Potato (Ipomoea batatas L.) Cultivars. Foods 2019, 8, 674. [Google Scholar] [CrossRef]
- Ramos, I.N.; Brandão, T.R.S.; Silva, C.L.M. Structural Changes During Air Drying of Fruits and Vegetables. Food Sci. Technol. Int. 2003, 9, 201–206. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Andrés, C.; Seguí, L.; Barrera, C.; Jiménez-Hernández, N.; Artacho, A.; Betoret, N.; Gosalbes, M.J. Valorization of Persimmon and Blueberry Byproducts to Obtain Functional Powders: In Vitro Digestion and Fermentation by Gut Microbiota. J. Agric. Food Chem. 2020, 68, 8080–8090. [Google Scholar] [CrossRef] [PubMed]
- Seguí, L.; Bas-Bellver, C.; Barrera, C.; Betoret, N. Valorization of Persimmon (Diospyros kaki) Wastes to Be Used as Functional Ingredients. In Mediterranean Fruits Bio-Wastes; Ramadan, M.F., Farag, M.A., Eds.; Springer Nature: Cham, Switzerland, 2022; pp. 739–764. [Google Scholar]
- Ramírez-Pulido, B.; Bas-Bellver, C.; Betoret, N.; Barrera, C.; Seguí, L. Valorization of Vegetable Fresh-Processing Residues as Functional Powdered Ingredients. A Review on the Potential Impact of Pretreatments and Drying Methods on Bioactive Compounds and Their Bioaccessibility. Front. Sustain. Food Syst. 2021, 5, 82. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Turning Agri-Food Cooperative Vegetable Residues into Functional Powdered Ingredients for the Food Industry. Sustainability 2020, 12, 1284. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Analytical Chemist Official Methods of Analysis. AOAC Official Method 934.06, Moisture in Dried Fruits, 17th ed.; Association of Official Analytical Chemist Official Methods of Analysis: Rockville, MD, USA, 2000. [Google Scholar]
- Mimouni, A.; Deeth, H.C.; Whittaker, A.K.; Gidley, M.J.; Bhandari, B.R. Rehydration process of milk protein concentrate powder monitored by static light scattering. Food Hydrocoll. 2009, 23, 1958–1965. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Corke, H. Production and Properties of Spray-dried Amaranthus Betacyanin Pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Freudig, B.; Hogekamp, S.; Schubert, H. Dispersion of powders in liquids in a stirred vessel. Chem. Eng. Process. 1999, 38, 525–532. [Google Scholar] [CrossRef]
- Raghavendra, S.N.; Ramachandra Swamy, S.R.; Rastogi, N.K.; Raghavarao, K.S.; Kumar, S.; Tharanathan, R.N. Grinding characteristics and hydration properties of coconut residue: A source of dietary fiber. J. Food Eng. 2006, 72, 281–286. [Google Scholar] [CrossRef]
- Garau, M.C.; Simal, S.; Rosselló, C.; Femenia, A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem. 2007, 104, 1014–1024. [Google Scholar] [CrossRef]
- Yasumatsu, K.; Sawada, K.; Moritaka, S.; Misaki, M.; Toda, J.; Wada, T.; Ishii, K. Whipping and Emulsifying Properties of Soybean Products. Food Nutr. (Roma) 1972, 36, 719–727. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Luximon-Ramma, A.; Bahorun, T.; Soobrattee, M.A.; Aruoma, O.I. Antioxidant Activities of Phenolic, Proanthocyanidin, and Flavonoid Components in Extracts of Cassia fistula. J. Agric. Food Chem. 2002, 50, 5042–5047. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Campas-Baypoli, O.N.; Sánchez-Machado, D.I.; Bueno-Solano, C.; Ramírez-Wong, B.; López-Cervantes, J. HPLC method validation for measurement of sulforaphane level in broccoli by-products. Biomed. Chromatogr. 2010, 24, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Doran, M.P. Bioprocess Engineering Principles, 2nd ed.; Elsevier: San Diego, CA, USA, 2013. [Google Scholar]
- Inyang, U.E.; Oboh, I.O.; Etuk, B.R.; Inyang, U.E.; Oboh, I.O.; Etuk, B.R. Kinetic Models for Drying Techniques—Food Materials. Adv. Chem. Eng. Sci. 2018, 8, 27–48. [Google Scholar] [CrossRef] [Green Version]
- Maskan, M. Drying, shrinkage and rehydration characteristics of kiwifruits during hot air and microwave drying. J. Food Eng. 2001, 48, 177–182. [Google Scholar] [CrossRef]
- Tello-Ireland, C.; Lemus-Mondaca, R.; Vega-Gálvez, A.; López, J.; di Scala, K. Influence of hot-air temperature on drying kinetics, functional properties, colour, phycobiliproteins, antioxidant capacity, texture and agar yield of alga Gracilaria chilensis. LWT-Food Sci. Technol. 2011, 44, 2112–2118. [Google Scholar] [CrossRef]
- Santos, P.H.S.; Silva, M.A. Retention of Vitamin C in Drying Processes of Fruits and Vegetables—A Review. Dry. Technol. 2008, 26, 1421–1437. [Google Scholar] [CrossRef]
- Mosquera, L.H.; Moraga, G.; Martínez-Navarrete, N. Critical water activity and critical water content of freeze-dried strawberry powder as affected by maltodextrin and arabic gum. Food Res. Int. 2012, 47, 201–206. [Google Scholar] [CrossRef]
- van Buggenhout, S.; Lille, M.; Messagie, I.; von Loey, A.; Autio, K.; Hendrickx, M. Impact of pretreatment and freezing conditions on the microstructure of frozen carrots: Quantification and relation to texture loss. Eur. Food Res. Technol. 2006, 222, 543–553. [Google Scholar] [CrossRef]
- Gulati, T.; Datta, A.K. Mechanistic understanding of case-hardening and texture development during drying of food materials. J. Food Eng. 2015, 166, 119–138. [Google Scholar] [CrossRef]
- Salvador, A.; Camacho, M.M.; Martínez-Navarrete, N. Influence of formulation on the quality and stability of a freeze-dried Mandarin product. Curr. Res. Food Sci. 2022, 5, 1047–1053. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, Y.; Lagnika, C.; Li, D.; Liu, C.; Jiang, N.; Song, J.; Zhang, M. A comparative evaluation of nutritional properties, antioxidant capacity and physical characteristics of cabbage (Brassica oleracea var. Capitate var L.) subjected to different drying methods. Food Chem. 2020, 309, 124935. [Google Scholar] [CrossRef]
- Vargas, L.; Kapoor, R.; Nemzer, B.; Feng, H. Application of different drying methods for evaluation of phytochemical content and physical properties of broccoli, kale, and spinach. LWT 2022, 155, 112892. [Google Scholar] [CrossRef]
- de Moraes Crizel, T.; Hermes, V.S.; de Oliveira Rios, A.; Flôres, S.H. Evaluation of bioactive compounds, chemical and technological properties of fruits byproducts powder. J. Food Sci. Technol. 2016, 53, 4067–4075. [Google Scholar] [CrossRef] [Green Version]
- Krokida, M.K.; Karathanos, V.T.; Maroulis, Z.B. Effect of freeze-drying conditions on shrinkage and porosity of dehydrated agricultural products. J. Food Eng. 1998, 35, 369–380. [Google Scholar] [CrossRef]
- Si, X.; Chen, Q.; Bi, J.; Wu, X.; Yi, J.; Zhou, L.; Li, Z. Comparison of different drying methods on the physical properties, bioactive compounds and antioxidant activity of raspberry powders. J. Sci. Food Agric. 2006, 96, 2055–2062. [Google Scholar] [CrossRef]
- Calabuig-Jiménez, L.; Indira Hinestroza-Córdoba, L.; Barrera, C.; Seguí, L.; Betoret, N. Effects of Processing and Storage Conditions on Functional Properties of Powdered Blueberry Pomace. Sustainability 2022, 14, 1839. [Google Scholar] [CrossRef]
- Shi, M.; Hlaing, M.M.; Ying, D.Y.; Ye, J.H.; Sanguansri, L.; Augustin, M.A. New food ingredients from broccoli by-products: Physical, chemical and technological properties. Int. J. Food Sci. Technol. 2019, 54, 1423–1432. [Google Scholar] [CrossRef]
- Kapoor, R.; Feng, H. Characterization of physicochemical, packing and microstructural properties of beet, blueberry, carrot and cranberry powders: The effect of drying methods. Powder Technol. 2022, 395, 290–300. [Google Scholar] [CrossRef]
- Forny, L.; Marabi, A.; Palzer, S. Wetting, disintegration and dissolution of agglomerated water soluble powders. Powder Technol. 2011, 206, 72–78. [Google Scholar] [CrossRef]
- Camacho, M.M.; Silva-Espinoza, M.A.; Martínez-Navarrete, N. Flowability, Rehydration Behaviour and bioactive Compounds of an Orange Powder Product as Affected by Particle Size. Food Bioprocess. Technol. 2022, 15, 683–692. [Google Scholar] [CrossRef]
- Olukomaiya, O.O.; Adiamo, O.Q.; Fernando, W.C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Effect of solid-state fermentation on proximate composition, anti-nutritional factor, microbiological and functional properties of lupin flour. Food Chem. 2020, 315, 126238. [Google Scholar] [CrossRef]
- Kethireddipalli, P.; Hung, Y.C.; Phillips, R.D.; McWatters, K.H. Evaluating the Role of Cell Wall Material and Soluble Protein in the Functionality of Cowpea (Vigna unguiculata) Pastes. J. Food Sci. 2002, 67, 53–59. [Google Scholar] [CrossRef]
- Jongaroontaprangsee, S.; Tritrong, W.; Chokanaporn, W.; Methacanon, P.; Devahastin, S.; Chiewchan, N. Effects of Drying Temperature and Particle Size on Hydration Properties of Dietary Fiber Powder from Lime and Cabbage By-Products. Int. J. Food Prop. 2007, 10, 887–897. [Google Scholar] [CrossRef]
- Que, F.; Mao, L.; Fang, X.; Wu, T. Comparison of hot air-drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Int. J. Food Sci. Technol. 2008, 43, 1195–1201. [Google Scholar] [CrossRef]
- Hinestroza-Córdoba, L.I.; Serna, S.D.; Seguí, L.; Barrera, C.; Betoret, N. Characterization of Powdered Lulo (Solanum quitoense) Bagasse as a Functional Food Ingredient. Foods 2020, 9, 723. [Google Scholar] [CrossRef]
- Ma, R.; Chen, J.; Zhou, X.; Lin, H.; Gao, Q.; Peng, X.; Tanokura, M.; Xue, Y. Effect of chemical and enzymatic modifications on the structural and physicochemical properties of dietary fiber from purple turnip (Brassica rapa L.). LWT 2021, 145, 111313. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.M.; Rodríguez-Gutiérrez, G.; Jaramillo-Carmona, S.; Espejo-Calvo, J.A.; Rodríguez-Arcos, R.; Fernández-Bolaños, J.; Guillén-Bejarano, R.; Jiménez-Araujo, A. Effect of extraction method on chemical composition and functional characteristics of high dietary fibre powders obtained from asparagus by-products. Food Chem. 2009, 113, 665–671. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. A comparative study on the polyphenolic content, antibacterial activity and antioxidant capacity of different solvent extracts of Brassica oleracea vegetables. Int. J. Food Sci. Technol. 2012, 47, 223–231. [Google Scholar] [CrossRef]
- Bernaert, N.; de Clercq, H.; van Bockstaele, E.; de Loose, M.; van Droogenbroeck, B. Antioxidant changes during postharvest processing and storage of leek (Allium ampeloprasum var. porrum). Postharvest Biol. Technol. 2013, 86, 8–16. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Effect of vacuum-drying, hot air-drying and freeze-drying on polyphenols and antioxidant capacity of lemon (Citrus limon) pomace aqueous extracts. Int. J. Food Sci. Technol. 2017, 52, 880–887. [Google Scholar] [CrossRef] [Green Version]
- Gahler, S.; Otto, K.; Böhm, V. Alterations of vitamin C, total phenolics, and antioxidant capacity as affected by processing tomatoes to different products. J. Agric. Food Chem. 2003, 51, 7962–7968. [Google Scholar] [CrossRef]
- Chen, M.L.; Yang, D.J.; Liu, S.C. Effects of drying temperature on the flavonoid, phenolic acid and antioxidative capacities of the methanol extract of citrus fruit (Citrus sinensis (L.) Osbeck) peels. Int. J. Food Sci. Technol. 2011, 46, 1179–1185. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-derived by-products—A promising source of bioactive ingredients. J. Food Sci. 2010, 75, 383–392. [Google Scholar] [CrossRef]
- Lekcharoenkul, P.; Tanongkankit, Y.; Chiewchan, N.; Devahastin, S. Enhancement of sulforaphane content in cabbage outer leaves using hybrid drying technique and stepwise change of drying temperature. J. Food Eng. 2014, 122, 56–61. [Google Scholar] [CrossRef]
- Tanongkankit, Y.; Chiewchan, N.; Devahastin, S. Evolution of anticarcinogenic substance in dietary fibre powder from cabbage outer leaves during drying. Food Chem. 2011, 127, 67–73. [Google Scholar] [CrossRef]
- van Eylen, D.; Oey, I.; Hendrickx, M.; van Loey, A. Kinetics of the stability of broccoli (Brassica oleracea Cv. Italica) myrosinase and isothiocyanates in broccoli juice during pressure/temperature treatments. J. Agric. Food Chem. 2007, 55, 2163–2170. [Google Scholar] [CrossRef] [PubMed]



| Sample | aw | Moisture Content (%) | xss (gss/gdm) |
|---|---|---|---|
| WC_G_HAD50 | 0.30 ± 0.04 bc | 4.3 ± 1.2 b | 0.62 ± 0.02 ab |
| WC_C_HAD50 | 0.285 ± 0.009 abc | 3.2 ± 0.9 ab | 0.637 ± 0.004 ab |
| WC_G_HAD60 | 0.256 ± 0.014 abc | 2.6 ± 1.4 ab | 0.652 ± 0.013 bc |
| WC_C_HAD60 | 0.254 ± 0.009 abc | 2.49 ± 0.12 ab | 0.61 ± 0.04 ab |
| WC_G_HAD70 | 0.23 ± 0.09 a | 3.1 ± 1.1 ab | 0.61 ± 0.06 ab |
| WC_C_HAD70 | 0.245 ± 0.08 ab | 2.6 ± 0.8 a | 0.58 ± 0.10 a |
| WC_G_FD | 0.30 ± 0.03 c | 3.4 ± 1.4 ab | 0.64 ± 0.05 bc |
| WC (raw) | 0.990 ± 0.003 d | 91.4 ± 0.2 c | 0.70 ± 0.02 c |
| B_G_HAD50 | 0.30 ± 0.04 ab | 3.7 ± 0.2 bc | 0.56 ± 0.03 a |
| B_C_HAD50 | 0.312 ± 0.08 b | 3.3 ± 0.2 ab | 0.60 ± 0.03 abc |
| B_G_HAD60 | 0.253 ± 0.007 a | 2.3 ± 0.7 a | 0.58 ± 0.02 ab |
| B_C_HAD60 | 0.283 ± 0.010 ab | 2.2 ± 0.64 a | 0.59 ± 0.02 b |
| B_G_HAD70 | 0.32 ± 0.05 ab | 3.8 ± 1.8 b | 0.59 ± 0.02 b |
| B_C_HAD70 | 0.30 ± 0.04 b | 2.6 ± 1.0 a | 0.61 ± 0.02 b |
| B_G_FD | 0.298 ± 0.04 ab | 2.7 ± 0.4 b | 0.608 ± 0.018 b |
| B (raw) | 0.998 ± 0.004 c | 92.83 ± 0.09 c | 0.70 ± 0.02 c |
| Sample | D[4,3] | D[3,2] | d10 | d50 | d90 |
|---|---|---|---|---|---|
| WC_G_HAD50 | 306 ± 12 c | 48.7 ± 1.1 d | 19.2 ± 0.4 c | 249 ± 7 d | 688 ± 33 cd |
| WC_C_HAD50 | 350 ± 49 d | 58 ± 4 f | 25 ± 2 e | 283 ± 38 e | 792 ± 117 e |
| WC_G_HAD60 | 292 ± 22 c | 51.8 ± 1.8 e | 20.7 ± 0.4 d | 240 ± 14 d | 650 ± 59 c |
| WC_C_HAD60 | 299 ± 24 c | 42.2 ± 0.7 b | 16.7 ± 0.2 b | 218 ± 13 c | 714 ± 66 d |
| WC_G_HAD70 | 231 ± 11 b | 47.0 ± 1.1 cd | 18.7 ± 0.6 c | 197 ± 9 b | 498 ± 29 a |
| WC_C_HAD70 | 294 ± 20 c | 45.7 ± 1.6 c | 16.9 ± 0.7 b | 241 ± 16 d | 661 ± 47 cd |
| WC_G_FD | 137 ± 3 a | 34.3 ± 0.6 a | 13.9 ± 0.2 a | 101 ± 2 a | 323 ± 8 a |
| B_G_HAD50 | 299 ± 13 b | 83 ± 4 b | 56 ± 3 b | 255 ± 8 b | 609 ± 36 b |
| B_C_HAD50 | 387 ± 37 d | 91 ± 5 c | 56 ± 3 b | 318 ± 27 c | 828 ± 94 d |
| B_G_HAD60 | 352 ± 18 c | 105 ± 2 e | 72.2 ± 1.9 f | 308 ± 10 c | 698 ± 50 c |
| B_C_HAD60 | 438 ± 34 e | 97 ± 3 d | 63 ± 2 d | 373 ± 23 d | 912 ± 83 e |
| B_G_HAD70 | 341 ± 22 c | 91 ± 4 c | 59 ± 3 c | 303 ± 15 c | 681 ± 51 c |
| B_C_HAD70 | 445 ± 38 e | 104 ± 4 e | 71 ± 4 e | 388 ± 28 d | 906 ± 91 e |
| B_G_FD | 145 ± 4 a | 42.4 ± 0.4 a | 18.3 ± 0.3 a | 102 ± 3 a | 341 ± 11 a |
| Sample | Chroma (Cab) | Hue (hab) | Sample | Chroma (Cab) | Hue (hab) |
|---|---|---|---|---|---|
| WC_G_HAD50 | 17.7 ± 0.2 bc | 82.9 ± 0.2 c | B_G_HAD50 | 20.7 ± 0.4 bc | 86.08 ± 0.05 c |
| WC_C_HAD50 | 18.3 ± 0.3 bc | 84.88 ± 0.05 d | B_C_HAD50 | 22.2 ± 0.4 c | 86.75 ± 0.16 c |
| WC_G_HAD60 | 16.7 ± 0.7 b | 83.39 ± 0.06 c | B_G_HAD60 | 20.1 ± 0.3 b | 86.76 ± 0.17 c |
| WC_C_HAD60 | 19.02 ± 0.04 c | 80.1 ± 0.2 b | B_C_HAD60 | 20.04 ± 0.02 b | 85.37 ± 0.15 c |
| WC_G_HAD70 | 19.71 ± 0.16 c | 80.5 ± 0.5 b | B_G_HAD70 | 17.2 ± 0.4 a | 82.6 ± 0.6 b |
| WC_C_HAD70 | 12 ± 3 a | 74.9 ± 1.1 a | B_C_HAD70 | 19.27 ± 0.16 b | 78 ± 3 a |
| WC_G_FD | 16.37 ± 0.09 b | 96.26 ± 0.14 e | B_G_FD | 24 ± 2 d | 94.7 ± 0.4 d |
| Sample | Spec. V (mL) | Solubility (%) | HG (%) | Wt (min) | SC (mL/g) | WHC (g/g) | WRC (g/g) | OHC (g/g) |
|---|---|---|---|---|---|---|---|---|
| WC_G_HAD50 | 1.80 ± 0.04 c | 52 ± 6 a | 63.0 ± 0.7 a | 8.7 ± 0.9 a | 11.17 ± 0.15 bc | 18 ± 3 ab | 9.0 ± 0.3 a | 3.87 ± 0.11 b |
| WC_C_HAD50 | 1.807 ± 0.012 c | 56 ± 4 a | 61.8 ± 0.7 a | 9 ± 2 a | 11.37 ± 0.06 c | 20 ± 7 ab | 15 ± 2 c | 3.1 ± 0.5 a |
| WC_G_HAD60 | 1.51 ± 0.02 a | 54 ± 3 a | 64.8 ± 0.6 ab | 14 ± 6 ab | 11.10 ± 0.10 b | 15 ± 6 a | 10.1 ± 0.7 ab | 4.2 ± 0.3 b |
| WC_C_HAD60 | 1.53 ± 0.02 a | 55 ± 6 a | 66.1 ± 1.2 ab | 18 ± 4 bc | 10.8 ± 0.3 a | 20 ± 4 ab | 9.9 ± 0.9 ab | 3.0 ± 0.4 a |
| WC_G_HAD70 | 1.993 ± 0.012 d | 55 ± 4 a | 68 ± 12 ab | 6 ± 2 a | 11.10 ± 0.11 b | 28 ± 5 b | 10 ± 2 ab | 4.38 ± 0.10 bc |
| WC_C_HAD70 | 1.67 ± 0.03 b | 55 ± 3 a | 67.1 ± 1.3 ab | 29 ± 4 c | 11.40 ± 0.10 c | 21 ± 6 ab | 11.0 ± 1.5 ab | 2.9 ± 0.2 a |
| WC_G_FD | 2.96 ± 0.03 e | 58 ± 4 a | 74 ± 11 b | 15 ± 6 ab | 10.8 ± 0.2 a | 21 ± 7 b | 13 ± 3 bc | 4.8 ± 0.5 c |
| B_G_HAD50 | 1.65 ± 0.04 c | 54 ± 4 a | 67.9 ± 0.7 ab | 2.5 ± 1.5 a | 10.70 ± 0.11 c | 33 ± 4 bc | 13.2 ± 1.6 b | 4.85 ± 0.14 c |
| B_C_HAD50 | 1.32 ± 0.02 a | 45 ± 3 a | 70.8 ± 0.6 ab | 4 ± 2 ab | 11.47 ± 0.06 f | 35 ± 10 c | 12.5 ± 1.3 b | 4.0 ± 0.5 b |
| B_G_HAD60 | 1.44 ± 0.04 b | 53 ± 11 a | 65.6 ± 0.5 a | 4.4 ± 1.2 ab | 10.93 ± 0.06 de | 24 ± 2 bc | 11.50 ± 0.14 b | 4.2 ± 0.3 b |
| B_C_HAD60 | 1.307 ± 0.012 a | 56 ± 6 a | 65.2 ± 0.6 a | 7.79 ± 1.16 bc | 10.91 ± 0.12 d | 25 ± 4 bc | 13 ± 2 b | 3.9 ± 0.3 b |
| B_G_HAD70 | 1.69 ± 0.06 c | 55 ± 4 a | 66 ± 3 a | 8.9 ± 0.6 c | 9.47 ± 0.06 b | 20 ± 4 ab | 7.7 ± 0.3 a | 3.3 ± 0.3 a |
| B_C_HAD70 | 1.27 ± 0.03 a | 52 ± 7 a | 77 ± 14 bc | 10 ± 5 c | 5.00 ± 0.10 a | 12 ± 3 a | 6.7 ± 0.5 a | 2.81 ± 0.15 a |
| B_G_FD | 3.98 ± 0.02 d | 54 ± 6 a | 84.3 ± 0.7 c | 1.9 ± 0.9 a | 11.03 ± 0.06 e | 32 ± 4 bc | 12.7 ± 0.7 b | 6.5 ± 0.3 d |
| Sample | Total Phenols (mg GAE/gdm) | Total Flavonoids (mg QE/gdm) | DPPH (mg TE/gdm) | ABTS (mg TE/gdm) |
|---|---|---|---|---|
| WC_G_HAD50 | 3.3 ± 0.2 a | 2.5 ± 0.7 a | 1.5 ± 0.3 ab | 49 ± 3 b |
| WC_C_HAD50 | 3.7 ± 0.8 a | 4.0 ± 1.0 cd | 3.0 ± 0.6 d | 51 ± 10 b |
| WC_G_HAD60 | 3.3 ± 0.5 a | 2.6 ± 0.7 ab | 1.3 ± 0.3 a | 49 ± 6 b |
| WC_C_HAD60 | 4.1 ± 1.1 ab | 3.7 ± 0.6 bc | 2.17 ± 0.14 c | 59.6 ± 1.2 b |
| WC_G_HAD70 | 4.8 ± 0.9 bc | 4.1 ± 1.2 cd | 1.4 ± 0.7 a | 48 ± 6 b |
| WC_C_HAD70 | 5.4 ± 0.4 c | 4.9 ± 0.6 d | 2.048 ± 0.016 abc | 58 ± 15 b |
| WC_G_FD | 4.0 ± 1.2 ab | 1.8 ± 0.4 a | 1.85 ± 0.14 abc | 25 ± 6 a |
| WC raw | 3.9 ± 0.4 a | 1.8 ± 0.3 a | 2.08 ± 0.17 bc | 30 ± 2 a |
| B_G_HAD50 | 3.5 ± 0.6 a | 2.2 ± 0.4 a | 2.60 ± 1.05 ab | 69 ± 4 b |
| B_C_HAD50 | 4.8 ± 0.5 b | 3.1 ± 0.2 b | 3.1 ± 0.4 bcd | 83 ± 11 c |
| B_G_HAD60 | 3.9 ± 0.6 ab | 2.6 ± 0.4 ab | 2.1 ± 0.3 a | 88 ± 11 cd |
| B_C_HAD60 | 4.4 ± 0.5 ab | 3.6 ± 0.8 c | 2.7 ± 0.5 abc | 98 ± 14 de |
| B_G_HAD70 | 6.7 ± 1.2 c | 5.3 ± 0.4 e | 2.4 ± 0.4 ab | 90 ± 10 cd |
| B_C_HAD70 | 8 ± 2 d | 8.1 ± 0.4 f | 3.40 ± 0.17 cd | 108 ± 8 e |
| B_G_FD | 4.3 ± 1.2 ab | 5.50 ± 0.03 e | 2.9 ± 0.9 bcd | 50 ± 5 a |
| B raw | 5.0 ± 1.0 b | 4.6 ± 0.7 d | 3.7 ± 0.3 d | 34.0 ± 1.6 a |
| Sample | White Cabbage (WC) | Broccoli (B) |
|---|---|---|
| G_HAD50 | 68.7 ± 0.5 ab | 470.2 ± 1.1 a |
| C_HAD50 | 67 ± 9 a | 479 ± 3 a |
| G_HAD60 | 71 ± 6 ab | 476 ± 10 a |
| C_HAD60 | 70 ± 6 ab | 472 ± 16 a |
| G_HAD70 | 69 ± 8 ab | 493 ± 52 a |
| C_HAD70 | 73 ± 3 ab | 461 ± 9 a |
| FD | 77 ± 4 ab | 506 ± 11 a |
| Raw | 105 ± 1 c | 787 ± 5 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Impact of Disruption and Drying Conditions on Physicochemical, Functional and Antioxidant Properties of Powdered Ingredients Obtained from Brassica Vegetable By-Products. Foods 2022, 11, 3663. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11223663
Bas-Bellver C, Barrera C, Betoret N, Seguí L. Impact of Disruption and Drying Conditions on Physicochemical, Functional and Antioxidant Properties of Powdered Ingredients Obtained from Brassica Vegetable By-Products. Foods. 2022; 11(22):3663. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11223663
Chicago/Turabian StyleBas-Bellver, Claudia, Cristina Barrera, Noelia Betoret, and Lucía Seguí. 2022. "Impact of Disruption and Drying Conditions on Physicochemical, Functional and Antioxidant Properties of Powdered Ingredients Obtained from Brassica Vegetable By-Products" Foods 11, no. 22: 3663. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11223663