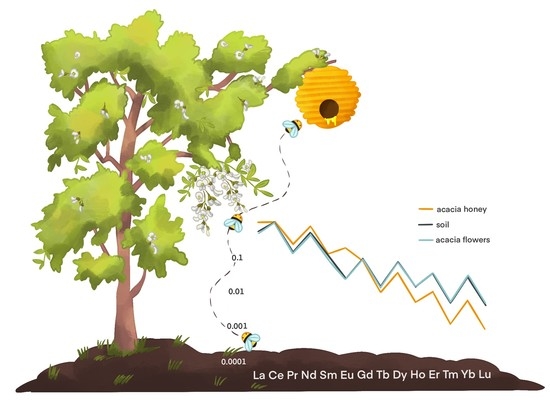
On the Traceability of Honey by Means of Lanthanide Distribution
Abstract
:1. Introduction
- Alpignano (province of Turin):
- Acacia;
- Taraxacum;
- Linden.
- Bagnasco (province of Cuneo):
- Chestnut.
- Borgo d’Ale (province of Vercelli):
- Acacia 2020;
- Acacia 2021;
- Chestnut 2020;
- Chestnut 2021, pollen.
- Cocconato (province of Asti):
- Acacia 2020;
- Acacia 2021;
- Linden.
- Colle del Lys (province of Turin):
- Clover 2020;
- Clover 2021.
- Giaveno (province of Turin):
- Chestnut.
- Montiglio (province of Asti):
- Acacia;
- Linden.
- Parco Colletta (province of Turin):
- Linden 2020;
- Linden 2021.
- Robilante (province of Cuneo):
- Chestnut/clover/taraxacum.
- Romano Canavese (province of Turin):
- Chestnut.
- Settimo Vittone (province of Turin):
- Clover.
2. Materials and Methods
2.1. Reagents
2.2. Sample Collection
2.3. Sample Treatment
2.3.1. Soil
2.3.2. Flowers
2.3.3. Honey
- Acid digestion was applied to 1.0 g of honey weighted directly into PTFE vessels, then 1 mL of 30% hydrogen peroxide, 2 mL of nitric acid 69% and 5 mL of HPW were added and the vessels were put inside the microwave oven system. The heating treatment increased the temperature from 25 °C to 180 °C in 15 min and kept the temperature constant for 10 min. The resulting solution was taken to 50 mL with HPW in a polypropylene tube. Solutions were then diluted 1:10 with HPW prior to ICP analysis.
- Dry ashing was applied by weighting ca. 15 g of honey into a porcelain capsule, then putting the capsule inside a Milestone (Sorisole, Italy) Pyro 260 microwave ashing system. The heating cycle was as follows: room temperature to 150 °C in 10’; hold at 150 °C for 20’; up to 500 °C in 20’; hold at 500 °C for 30’; up to 750 °C in 10’; hold at 750 °C for 30’; up to 1000 °C in 10’; hold at 1000 °C for 30’. The resulting ash was completely dissolved in 2.0 mL of ultrapure concentrated nitric acid and taken up to 50 mL with HPW in a polypropylene tube. Solutions were then diluted 1:10 with HPW prior to ICP analysis.
2.4. ICP-MS Analysis
2.5. ICP-OES Analysis
2.6. Analysis of Certified Samples
2.7. Data Analysis
3. Results
3.1. Traceability of the Honey Chain
3.2. Different Floral Species on the Same Soil
3.3. Different Floral Species in the Same Honey
3.4. Different Years
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zappi, A.; Melucci, D.; Scaramagli, S.; Zelano, A.; Marcazzan, G.L. Botanical Traceability of Unifloral Honeys by Chemometrics Based on Head-Space Gas Chromatography. Eur. Food Res. Technol. 2018, 244, 2149–2157. [Google Scholar] [CrossRef]
- CXS 12-1981; Codex Alimentarius Commission Standard for Honey CXS 12-1981. WHO: Geneva, Switzerland, 2019.
- EU Council. Council Directive 2001/110/EC of 20 December 2001 Relating to Honey. Off. J. Eur. Communities 2002, L10, 47–52. [Google Scholar]
- Moore, J.C.; Spink, J.; Lipp, M. Development and Application of a Database of Food Ingredient Fraud and Economically Motivated Adulteration from 1980 to 2010. J. Food Sci. 2012, 77, R118–R126. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P. Determination of Metal Content in Honey by Atomic Absorption and Emission Spectrometries. TrAC—Trends Anal. Chem. 2009, 28, 117–128. [Google Scholar] [CrossRef]
- Magdas, D.A.; Guyon, F.; Puscas, R.; Vigouroux, A.; Gaillard, L.; Dehelean, A.; Feher, I.; Cristea, G. Applications of Emerging Stable Isotopes and Elemental Markers for Geographical and Varietal Recognition of Romanian and French Honeys. Food Chem. 2021, 334, 127599. [Google Scholar] [CrossRef]
- Sabatini, A.G.; Bortolotti, L.; Marcazzan, G.L. Conoscere Il Miele; Edizioni Avenue Media: Bologna-Milano, Italy, 2007. [Google Scholar]
- Lestari, L.A.; Triyana, K.; Hanifah, A.K.; Wildiana, R.A. The Use of Electronic Tongue (e-Tongue) as a Simple and Rapid Method for Honey Authentication. Food Res. 2021, 5, 453–460. [Google Scholar] [CrossRef]
- Martin, P. Importance of Melissopalynology for Beekeeping and Trade. Bee World 2005, 86, 75–76. [Google Scholar] [CrossRef]
- Corvucci, F.; Nobili, L.; Melucci, D.; Grillenzoni, F.V. The Discrimination of Honey Origin Using Melissopalynology and Raman Spectroscopy Techniques Coupled with Multivariate Analysis. Food Chem. 2015, 169, 297–304. [Google Scholar] [CrossRef]
- Davies, A.M.C.; Radovic, B.; Fearn, T.; Anklam, E. A Preliminary Study on the Characterisation of Honey by near Infrared Spectroscopy. J. Near Infrared Spectrosc. 2017, 10, 121–135. [Google Scholar] [CrossRef]
- Tan, S.H.; Pui, L.P.; Solihin, M.I.; Keat, K.S.; Lim, W.H.; Ang, C.K. Physicochemical Analysis and Adulteration Detection in Malaysia Stingless Bee Honey Using a Handheld Near-infrared Spectrometer. J. Food Process. Preserv. 2021, 45, e15576. [Google Scholar] [CrossRef]
- Berghian-Grosan, C.; Hategan, A.R.; David, M.; Magdas, D.A. Untargeted Metabolomic Analysis of Honey Mixtures: Discrimination Opportunities Based on ATR-FTIR Data and Machine Learning Algorithms. Microchem. J. 2023, 188, 108458. [Google Scholar] [CrossRef]
- Sahlan, M.; Ahlam, N.A.L.; Agus, A.; Sabir, A.; Pratami, D.K. Identification and Authentication of Honey Using Chemometric Analysis Based on ATR-FTIR and Raman Spectroscopy. Int. J. Appl. Pharm. 2022, 14, 36–44. [Google Scholar] [CrossRef]
- Sotiropoulou, N.S.; Xagoraris, M.; Revelou, P.K.; Kaparakou, E.; Kanakis, C.; Pappas, C.; Tarantilis, P. The Use of SPME-GC-MS IR and Raman Techniques for Botanical and Geographical Authentication and Detection of Adulteration of Honey. Foods 2021, 10, 1671. [Google Scholar] [CrossRef] [PubMed]
- Escriche, I.; Kadar, M.; Domenech, E.; Gil-Sánchez, L. A Potentiometric Electronic Tongue for the Discrimination of Honey According to the Botanical Origin. Comparison with Traditional Methodologies: Physicochemical Parameters and Volatile Profile. J. Food Eng. 2012, 109, 449–456. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Badeka, A.V.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Botanical Discrimination of Greek Unifloral Honeys with Physico-Chemical and Chemometric Analyses. Food Chem. 2014, 165, 181–190. [Google Scholar] [CrossRef]
- Ye, H.; Yang, J.; Xiao, G.; Zhao, Y.; Li, Z.; Bai, W.; Zeng, X.; Dong, H. A Comprehensive Overview of Emerging Techniques and Chemometrics for Authenticity and Traceability of Animal-Derived Food. Food Chem. 2023, 402, 134216. [Google Scholar] [CrossRef]
- Danieli, P.P.; Lazzari, F. Honey Traceability and Authenticity. Review of Current Methods Most Used to Face This Problem. J. Apic. Sci. 2022, 66, 101–119. [Google Scholar] [CrossRef]
- Zhang, G.; Abdulla, W. On Honey Authentication and Adulterant Detection Techniques. Food Control 2022, 138, 108992. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I.; Rahman, A. Application of Analytical Methods in Authentication and Adulteration of Honey. Food Chem. 2017, 217, 687–698. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Koulis, G.A.; Danezis, G.P.; Martakos, I.; Dasenaki, M.; Georgiou, C.A.; Thomaidis, N.S. Honey Authenticity: Analytical Techniques, State of the Art and Challenges. RSC Adv. 2021, 11, 11273–11294. [Google Scholar] [CrossRef]
- Aceto, M. The Use of ICP-MS in Food Traceability. In Advances in Food Traceability Techniques and Technologies; Espiñeira, M., Santaclara, F.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 137–164. [Google Scholar]
- Mazarakioti, E.C.; Zotos, A.; Thomatou, A.-A.; Kontogeorgos, A.; Patakas, A.; Ladavos, A. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS), a Useful Tool in Authenticity of Agricultural Products’ and Foods’ Origin. Foods 2022, 11, 3705. [Google Scholar] [CrossRef]
- Drivelos, S.A.; Danezis, G.P.; Halagarda, M.; Popek, S.; Georgiou, C.A. Geographical Origin and Botanical Type Honey Authentication through Elemental Metabolomics via Chemometrics. Food Chem. 2021, 338, 127936. [Google Scholar] [CrossRef]
- Voica, C.; Iordache, A.M.; Ionete, R.E. Multielemental Characterization of Honey Using Inductively Coupled Plasma Mass Spectrometry Fused with Chemometrics. J. Mass Spectrom. 2020, 55, e4512. [Google Scholar] [CrossRef]
- Weilert, T.M.; Ray, C.L.; Gawenis, J.A.; Brockman, J.D. Neutron Activation Analysis and ICP-MS for Provenance of Honey Collected from American Midwest Region. J. Radioanal. Nucl. Chem. 2022, 331, 4971–4981. [Google Scholar] [CrossRef]
- Oddone, M.; Aceto, M.; Baldizzone, M.; Musso, D.; Osella, D. Authentication and Traceability Study of Hazelnuts from Piedmont, Italy. J. Agric. Food Chem. 2009, 57, 3404–3408. [Google Scholar] [CrossRef]
- Aceto, M.; Calà, E.; Musso, D.; Regalli, N.; Oddone, M. A Preliminary Study on the Authentication and Traceability of Extra Virgin Olive Oil Made from Taggiasca Olives by Means of Trace and Ultra-Trace Elements Distribution. Food Chem. 2019, 298, 125047. [Google Scholar] [CrossRef]
- Aceto, M.; Musso, D.; Calà, E.; Arieri, F.; Oddone, M. Role of Lanthanides in the Traceability of the Milk Production Chain. J. Agric. Food Chem. 2017, 65, 4200–4208. [Google Scholar] [CrossRef]
- Aceto, M.; Gulino, F.; Calà, E.; Robotti, E.; Petrozziello, M.; Tsolakis, C.; Cassino, C. Authentication and Traceability Study on Barbera d’asti and Nizza Docg Wines: The Role of Trace-and Ultra-Trace Elements. Beverages 2020, 6, 63. [Google Scholar] [CrossRef]
- Aceto, M.; Robotti, E.; Oddone, M.; Baldizzone, M.; Bonifacino, G.; Bezzo, G.; Di Stefano, R.; Gosetti, F.; Mazzucco, E.; Manfredi, M.; et al. A Traceability Study on the Moscato Wine Chain. Food Chem. 2013, 138, 1914–1922. [Google Scholar] [CrossRef]
- Somanathan, H.; Saryan, P.; Balamurali, G.S. Foraging Strategies and Physiological Adaptations in Large Carpenter Bees. J. Comp. Physiol. A 2019, 205, 387–398. [Google Scholar] [CrossRef]
- Calà, E.; Fracchia, A.; Robotti, E.; Gulino, F.; Gullo, F.; Oddone, M.; Massacane, M.; Cordone, G.; Aceto, M. On the Traceability of the Hazelnut Production Chain by Means of Trace Elements. Molecules 2022, 27, 3854. [Google Scholar] [CrossRef] [PubMed]
- Oddo, G. Die Molekularstruktur Der Radioaktiven Atome. Z. Anorg. Chem. 1914, 87, 253–268. [Google Scholar] [CrossRef]
- Brown, P.H.; Rathjen, A.H.; Graham, R.D.; Tribe, D.E. Rare Earth Elements in Biological Systems. In Handbook on the Physics and Chemistry of Rare Earths; Gschneider, K.A., Eyring, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 423–452. [Google Scholar]
- Tyler, G. Rare Earth Elements in Soil and Plant Systems—A Review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Liang, T.; Ding, S.; Song, W.; Chong, Z.; Zhang, C.; Li, H. A Review of Fractionations of Rare Earth Elements in Plants. J. Rare Earths 2008, 26, 7–15. [Google Scholar] [CrossRef]







| Element | Certified Values (mg/kg) | Uncertainty | Found (mg/kg) | r.s.d. (%) |
|---|---|---|---|---|
| Li | 25 1 | 23.3 | 3.0 | |
| Be | 1.4 1 | 0.82 | 5.4 | |
| Na | 4680 | 730 | 2140 | 3.5 |
| Mg | 17,070 | 840 | 7294 | 1.2 |
| Al | 66,520 | 760 | 37,443 | 2.1 |
| P | 1001 | 77 | 1185 | 5.6 |
| K | 9760 | 180 | 3255 | 2.1 |
| Ca | 22,180 | 540 | 15,637 | 2.2 |
| Ti | 6050 | 660 | 5065 | 1.6 |
| V | 160 1 | 122 | 1.3 | |
| Cr | 301 | 45 | 141 | 3.4 |
| Mn | 1000 | 18 | 861 | 1.7 |
| Fe | 51,610 | 890 | 44,309 | 0.9 |
| Co | 35 1 | 23 | 0.9 | |
| Ni | 75 1 | 45 | 2.3 | |
| Cu | 81 1 | 55 | 2.9 | |
| Zn | 352 | 16 | 263 | 1.5 |
| As | 8.7 | 1.5 | 7.0 | 2.2 |
| Se | 0.6 1 | 2.1 | 5.0 | |
| Y | 21 1 | 15 | 1.2 | |
| Rb | 2 | 799 | 1.4 | |
| Sr | 84.1 | 8.0 | 34.1 | 2.0 |
| Nb | 6 1 | 1.1 | 2.3 | |
| Cd | 2.71 | 0.54 | 2.28 | 0.8 |
| Ba | 413 | 18 | 286 | 1.3 |
| La | 29.7 | 4.8 | 23.9 | 1.7 |
| Ce | 58 | 8 | 47 | 1.3 |
| Pr | 7.3 1 | 6.0 | 2.8 | |
| Nd | 26.4 | 2.9 | 22.4 | 2.9 |
| Sm | 6.1 1 | 4.5 | 3.3 | |
| Eu | 1.5 1 | 0.9 | 3.6 | |
| Gd | 5.8 1 | 4.0 | 2.1 | |
| Tb | 0.9 1 | 0.6 | 2.2 | |
| Dy | 5.4 1 | 3.2 | 1.6 | |
| Ho | 1.1 1 | 0.6 | 3.2 | |
| Er | 3.3 1 | 1.6 | 2.0 | |
| Tm | 0.5 1 | 0.2 | 1.3 | |
| Yb | 2.64 | 0.51 | 1.34 | 1.3 |
| Lu | 2 | 0.2 | 3.9 | |
| Pb | 432 | 17 | 365 | 3.3 |
| Th | 7 1 | 5.7 | 5.5 |
| Element | Certified Values (mg/kg) | Uncertainty | Found (mg/kg) | r.s.d. (%) |
|---|---|---|---|---|
| Li | 2 | 0.83 | 5.4 | |
| Be | 2 | 0.036 | 9.4 | |
| Na | 136 | 4 | 79 | 1.3 |
| Mg | 12,000 1 | 10,150 | 2.1 | |
| Al | 598 | 12 | 392 | 3.4 |
| Si | 2 | 145 | 5.2 | |
| P | 2160 | 40 | 1969 | 6.3 |
| S | 9600 1 | 10,478 | 4.7 | |
| K | 27,000 | 500 | 15,463 | 1.4 |
| Ca | 50,500 | 900 | 59,548 | 2.4 |
| Ti | 2 | 5.73 | 10.0 | |
| V | 0.835 | 0.010 | 0.222 | 3.3 |
| Cr | 1.99 | 0.06 | 0.61 | 4.0 |
| Mn | 246 | 8 | 245 | 3.2 |
| Fe | 368 | 7 | 224 | 2.6 |
| Co | 0.57 | 0.02 | 0.74 | 1.4 |
| Ni | 1.59 | 0.07 | 1.20 | 1.8 |
| Cu | 4.70 | 0.14 | 6.92 | 2.0 |
| Zn | 30.9 | 0.7 | 65.8 | 1.6 |
| As | 0.112 | 0.004 | 0.160 | 5.2 |
| Se | 0.054 | 0.003 | 0.139 | 31.9 |
| Rb | 14.89 | 0.27 | 22.35 | 2.3 |
| Sr | 85 1 | 58 | 1.2 | |
| Y | 2 | 0.94 | 1.0 | |
| Zr | 2 | 0.33 | 1.6 | |
| Mo | 0.46 1 | 0.46 | 0.5 | |
| Cd | 1.52 | 0.04 | 1.62 | 1.7 |
| Ba | 63 1 | 56 | 2.0 | |
| La | 2.3 1 | 2.2 | 1.8 | |
| Ce | 2 1 | 2.0 | 0.8 | |
| Pr | 2 | 0.37 | 2.1 | |
| Nd | 2 | 1.2 | 3.5 | |
| Sm | 0.19 1 | 0.18 | 3.6 | |
| Eu | 2 | 0.035 | 0.5 | |
| Gd | 0.17 1 | 0.15 | 4.4 | |
| Tb | 2 | 0.021 | 2.6 | |
| Dy | 2 | 0.097 | 1.3 | |
| Ho | 2 | 0.020 | 2.9 | |
| Er | 2 | 0.045 | 2.2 | |
| Tm | 2 | 0.005 | 4.5 | |
| Yb | 2 | 0.025 | 2.4 | |
| Lu | 2 | 0.004 | 6.2 | |
| Pb | 2 | 0.976 | 1.9 | |
| Th | 0.12 1 | 0.03 | 1.9 | |
| U | 0.035 1 | 0.022 | 5.0 |
| Element | Soils | Flowers | Honeys | |||
|---|---|---|---|---|---|---|
| Min–Max | Average | Min–Max | Average | Min–Max | Average | |
| Li | 5.75–68.7 | 31.8 | 0.015–2.05 | 0.371 | 0.103–3.19 | 0.653 |
| Be | 0.181–1.07 | 0.626 | 0.000401–0.0518 | 0.009442 | 0.000026–0.001705 | 0.000266 |
| B | 124–348 | 133 | 1–23.8 | 1.71 | 1–3.29 | |
| Na | 102–2140 | 1344 | 6.76–272 | 64.6 | 0.979–43.5 | 15.7 |
| Mg | 9.85–14673 | 8158 | 874–5359 | 2918 | 0.093–11.7 | 2.69 |
| Al | 2 | 2 | 17.9–842 | 187 | 0.146–22.7 | 5.27 |
| Si | 2 | 2 | 9.16–549 | 148 | 0.340–14.9 | 2.90 |
| P | 514–5907 | 1531 | 1159–6429 | 3681 | 3.80–59.0 | 13.2 |
| S | 765–11,886 | 2727 | 800–4791 | 2858 | 1.58–53.2 | 8.01 |
| K | 969–7758 | 2587 | 4947–25,711 | 15,457 | 2.14–134 | 31.6 |
| Ca | 30.9–198,804 | 45,253 | 1572–27,171 | 12,575 | 0.266–44.4 | 23.1 |
| Ti | 134–6319 | 1987 | 0.449–26.6 | 6.34 | 0.006199–0.0808 | 0.0231 |
| V | 6.07–122 | 59.4 | 0.0131–1.63 | 0.263 | 0.001211–0.005467 | 0.002616 |
| Cr | 6.42–246 | 99.3 | 0.0738–2.75 | 0.773 | 0.000475–0.0112 | 0.004419 |
| Mn | 0.82–2430 | 730 | 11.1–181 | 62.4 | 0.000294–1.221 | 0.117 |
| Fe | 65.3–56,229 | 28,181 | 54.2–1341 | 249 | 0.0769–1.675 | 0.388 |
| Co | 1.04–25.9 | 13.4 | 0.0372–1.05 | 0.241 | 0.000115–0.002056 | 0.000531 |
| Ni | 3.87–143 | 69.5 | 0.964–26.8 | 8.81 | 0.001935–0.0325 | 0.007813 |
| Cu | 4.93–131 | 55.4 | 8.43–20.9 | 13.9 | 0.0101–0.312 | 0.0612 |
| Zn | 31.6–263 | 81.6 | 19.0–97.9 | 48.3 | 0.0138–0.195 | 0.0629 |
| As | 2.57–11.0 | 5.70 | 0.007002–1.29 | 0.176 | 0.000349–0.001607 | 0.000788 |
| Se | 0.645–3.99 | 1.74 | 0.005161–0.135 | 0.0529 | 0.000084–0.001401 | 0.000462 |
| Rb | 739–2325 | 1113 | 0.77–78.2 | 36.8 | 1–23.3 | 0.907 |
| Sr | 8.98–517 | 93.0 | 1.101–120 | 27.4 | 0.0752–0.424 | 0.191 |
| Y | 3.00–30.3 | 14.0 | 0.008261–2.26 | 0.255 | 0.000310–0.004471 | 0.001272 |
| Zr | 0.022424–10.1 | 0.635 | 0.002815–0.329 | 0.0852 | 0.000423–0.0149 | 0.002568 |
| Nb | 0.012129–4.27 | 1.09 | 1–1 | 1 | 0.000064–0.000417 | 0.000151 |
| Mo | 0.545–3.35 | 1.90 | 0.0414–11.9 | 3.17 | 0.001453–0.008853 | 0.004012 |
| Cd | 0.111–2.28 | 0.295 | 0.000012–0.148 | 0.0487 | 0.000008–0.000121 | 0.000039 |
| Sb | 0.174–2.05 | 0.538 | 0.000759–0.0755 | 0.0390 | 0.000007–0.000142 | 0.000029 |
| Ba | 0.75–356 | 155 | 0.969–64.9 | 16.0 | 0.0075–0.733 | 0.410 |
| La | 5.90–36.6 | 19.0 | 0.008276–1.36 | 0.257 | 0.002922–0.0196 | 0.009173 |
| Ce | 12.7–64.8 | 36.8 | 0.0188–1.73 | 0.383 | 0.003596–0.0317 | 0.0107 |
| Pr | 1.51–9.18 | 4.70 | 0.001485–0.214 | 0.0480 | 0.000645–0.005398 | 0.002205 |
| Nd | 6.02–34.3 | 17.5 | 0.005083–0.795 | 0.172 | 0.002079–0.0190 | 0.007551 |
| Sm | 1.45–8.13 | 3.75 | 0.000921–0.187 | 0.0352 | 0.000334–0.003780 | 0.001317 |
| Eu | 0.166–2.02 | 0.772 | 0.000431–0.0402 | 0.008415 | 0.000771–0.003210 | 0.001644 |
| Gd | 1.32–7.70 | 3.44 | 0.001285–0.198 | 0.0370 | 0.000203–0.002514 | 0.000842 |
| Tb | 0.180–1.20 | 0.546 | 0.000200–0.0297 | 0.005955 | 0.000027–0.000365 | 0.000114 |
| Dy | 0.785–6.22 | 2.88 | 0.001274–0.159 | 0.0305 | 0.000105–0.001568 | 0.000458 |
| Ho | 0.130–1.30 | 0.581 | 0.000269–0.0380 | 0.006381 | 0.000015–0.000222 | 0.000064 |
| Er | 0.295–3.39 | 1.50 | 0.000675–0.0903 | 0.0154 | 0.000029–0.000470 | 0.000131 |
| Tm | 0.0368–0.513 | 0.219 | 0.000098–0.0106 | 0.002015 | 0.000004–0.000061 | 0.000016 |
| Yb | 0.194–2.95 | 1.24 | 0.000571–0.0514 | 0.0103 | 0.000019–0.000335 | 0.000085 |
| Lu | 0.0277–0.476 | 0.193 | 0.000091–0.007972 | 0.001665 | 0.000002–0.000050 | 0.000012 |
| W | 0.0216–1.49 | 0.357 | 1–0.418 | 1 | 0.001962–0.0952 | 0.0204 |
| Tl | 0.0855–0.685 | 0.276 | 0.000541–0.0799 | 0.0129 | 0.000002–0.000730 | 0.000061 |
| Pb | 12.3–365 | 39.9 | 0.0794–3.88 | 0.646 | 0.001545–0.0206 | 0.004516 |
| Th | 1.18–12.2 | 5.94 | 0.001084–0.118 | 0.0196 | 0.000221–0.004136 | 0.000883 |
| U | 0.373–4.26 | 1.58 | 0.000972–0.0541 | 0.0103 | 0.000047–0.001480 | 0.000218 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulino, F.; Calà, E.; Cozzani, C.; Vaccari, L.; Oddone, M.; Aceto, M. On the Traceability of Honey by Means of Lanthanide Distribution. Foods 2023, 12, 1803. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12091803
Gulino F, Calà E, Cozzani C, Vaccari L, Oddone M, Aceto M. On the Traceability of Honey by Means of Lanthanide Distribution. Foods. 2023; 12(9):1803. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12091803
Chicago/Turabian StyleGulino, Federica, Elisa Calà, Christian Cozzani, Lorenzo Vaccari, Matteo Oddone, and Maurizio Aceto. 2023. "On the Traceability of Honey by Means of Lanthanide Distribution" Foods 12, no. 9: 1803. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12091803