Effect of Wild Strawberry Tree and Hawthorn Extracts Fortification on Functional, Physicochemical, Microbiological, and Sensory Properties of Yogurt
Abstract
:1. Introduction
2. Materials and Methods
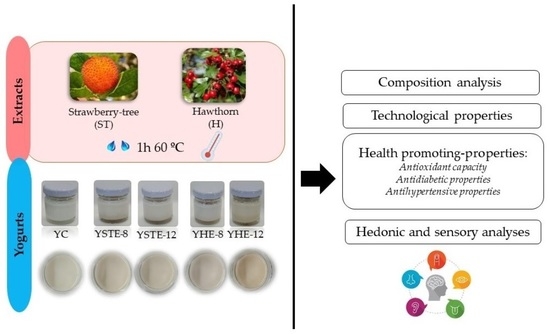
2.1. Raw Materials and Extracts
2.2. Yogurt Preparations
2.3. Composition Analyses
2.4. Health-Promoting Properties
- 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assay: The trapping capacity of cationic free radicals was evaluated using the method of radical ABTS•+ bleaching [25,26]. Aqueous solutions of Trolox (0.02–1 mM) were used for calibration. Absorbance was measured at 734 nm in an automated plate reader (BMG LABTECH, Ortenberg, Germany). All measurements were performed in triplicate, and results were expressed as µmol TE/g sample;
- Oxygen Radical Absorbance Capacity (ORAC) assay: The ORAC assay was applied according to the method using fluorescein as a fluorescence probe [27]. The procedure was carried out using an automated plate reader (BMG LABTECH, Ortenberg, Germany) equipped with a fluorescence detector set at excitation and emission wavelengths of 485 nm and 530 nm, respectively. Readings were taken every minute for 90 min at 37 °C. All measurements were performed in triplicate, and results were expressed as µmol TE/g sample.
- α-amylase inhibition assay: The inhibitory activity of extracts and yogurts against α-amylase was measured using starch as substrate for the Caraway-Somogyi iodine/potassium iodide method [28,29] with slight modifications adapted to microplate [30]. Results were expressed as percentages of α-amylase inhibition. All measurements were performed in triplicate. Acarbose was used as a positive control;
- α-glucosidase inhibition assay: The α-glucosidase inhibitory activity of wild fruit extracts and yogurts was analyzed [31,32,33]. Acarbose was used as a positive control (standard inhibitor). Results were expressed as a percentage of α-glucosidase inhibition. All measurements were performed in triplicate;
- Lipase inhibition assay: The inhibitory activity of extracts and yogurt samples against pancreatic lipase was measured by using 4-methylumbelliferyl oleate (4-MUO) as substrate [34,35]. Orlistat was used as a positive control. Results were expressed as a percentage of lipase inhibition. All measurements were performed in triplicate.
2.5. Technological Properties
2.6. Hedonic and Sensory Analyses
2.7. Statistical Analyses
3. Results and Discussion
3.1. Characterization of Wild Fruit Extracts: STE and HE
3.1.1. Composition of Wild Fruit Extracts
3.1.2. Health Promoting Properties of Wild Fruit Extracts
3.1.3. Technological Properties of Wild Fruit Extracts
3.2. Application of Wild Fruit Extracts as Food Ingredients in Yogurt
3.2.1. Composition of Yogurts Containing Wild Fruit Extracts
3.2.2. Health Promoting Properties of Yogurts Containing Wild Fruit Extracts
3.2.3. Technological Properties of Yogurts Containing Wild Fruit Extracts
3.2.4. Hedonic and Sensory Analyses of Yogurts Containing Wild Fruit Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heinrich, M.; Nebel, S.; Leonti, M.; Rivera, D.; Obón, C. ‘Local Food-Nutraceuticals’: Bridging the Gap between Local Knowledge and Global Needs. In Local Mediterranean Food Plants and Nutraceuticals; KARGER: Basel, Switzerland, 2006; Volume 59, pp. 1–17. [Google Scholar]
- Ali, S.S.; Kasoju, N.; Luthra, A.; Singh, A.; Sharanabasava, H.; Sahu, A.; Bora, U. Indian Medicinal Herbs as Sources of Antioxidants. Food Res. Int. 2008, 41, 1–15. [Google Scholar] [CrossRef]
- Pinela, J.; Carvalho, A.M.; Ferreira, I.C. Wild Edible Plants: Nutritional and Toxicological Characteristics, Retrieval Strategies and Importance for Today’s Society. Food Chem. Toxicol. 2017, 110, 165–188. [Google Scholar] [CrossRef] [PubMed]
- de Cortes Sánchez-Mata, M.; Tardío, J. Mediterranean Wild Edible Plants: Ethnobotany and Food Composition Tables; Springer: New York, USA, 2016; ISBN 9781493933297. [Google Scholar]
- Trichopoulou, A.; Vasilopoulou, E.; Hollman, P.; Chamalides, C.; Foufa, E.; Kaloudis, T.; Kromhout, D.; Miskaki, P.; Petrochilou, I.; Poulima, E.; et al. Nutritional Composition and Flavonoid Content of Edible Wild Greens and Green Pies: A Potential Rich Source of Antioxidant Nutrients in the Mediterranean Diet. Food Chem. 2000, 70, 319–323. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, B.M.; Morales, P.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Cámara, M.; Díez-Marqués, C.; Pardo-de-Santayana, M.; Molina, M.; Tardío, J. Valorization of Wild Strawberry-Tree Fruits (Arbutus unedo L.) through Nutritional Assessment and Natural Production Data. Food Res. Int. 2011, 44, 1244–1253. [Google Scholar] [CrossRef]
- Alarcão-E-Silva, M.; Leitão, A.E.B.; Azinheira, H.G.; Leitão, M.C.A. The Arbutus Berry: Studies on Its Color and Chemical Characteristics at Two Mature Stages. J. Food Compos. Anal. 2001, 14, 27–35. [Google Scholar] [CrossRef]
- Ganhão, R.; Estévez, M.; Kylli, P.; Heinonen, M.; Morcuende, D. Characterization of Selected Wild Mediterranean Fruits and Comparative Efficacy as Inhibitors of Oxidative Reactions in Emulsified Raw Pork Burger Patties. J. Agric. Food Chem. 2010, 58, 8854–8861. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodríguez, B.M.; de Ancos, B.; Sánchez-Moreno, C.; Fernández-Ruiz, V.; de Cortes Sánchez-Mata, M.; Cámara, M.; Tardío, J. Wild Blackthorn (Prunus spinosa L.) and Hawthorn (Crataegus monogyna Jacq.) Fruits as Valuable Sources of Antioxidants. Fruits 2014, 69, 61–73. [Google Scholar] [CrossRef]
- Morales, D. Use of Strawberry Tree (Arbutus unedo) as a Source of Functional Fractions with Biological Activities. Foods 2022, 11, 3838. [Google Scholar] [CrossRef]
- Fazel Nabavi, S.; Habtemariam, S.; Ahmed, T.; Sureda, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.M. Polyphenolic Composition of Crataegus monogyna Jacq.: From Chemistry to Medical Applications. Nutrients 2015, 7, 7708–7728. [Google Scholar] [CrossRef]
- Bahorun, T.; Aumjaud, E.; Ramphul, H.; Rycha, M.; Luximon-Ramma, A.; Trotin, F.; Aruoma, O.I. Phenolic Constituents and Antioxidant Capacities of Crataegus monogyna (Hawthorn) Callus Extracts. Nahr. Food 2003, 47, 191–198. [Google Scholar] [CrossRef]
- Muresan, A.E.; Muste, S.; Petrut, G.; Vlaic, R.A.; Man, S.M.; Muresan, V. New Uses of Hawthorn Fruits in Tonic Wines Technology. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca. Food Sci. Technol. 2016, 73, 117. [Google Scholar] [CrossRef] [PubMed]
- Dabija, A.; Codinǎ, G.G.; Ropciuc, S.; Gâtlan, A.M.; Rusu, L. Assessment of the Antioxidant Activity and Quality Attributes of Yogurt Enhanced with Wild Herbs Extracts. J. Food Qual. 2018, 2018, 5329386. [Google Scholar] [CrossRef]
- Weerathilake, W.A.D.V.; Rasika, D.M.D.; Ruwanmali, J.K.U.; Munasinghe, M.A.D.D. The Evolution, Processing, Varieties and Health Benefits of Yogurt. Int. J. Sci. Res. Publ. 2014, 4, 1–10. [Google Scholar]
- Granato, D.; Santos, J.S.; Salem, R.D.; Mortazavian, A.M.; Rocha, R.S.; Cruz, A.G. Effects of Herbal Extracts on Quality Traits of Yogurts, Cheeses, Fermented Milks, and Ice Creams: A Technological Perspective. Curr. Opin. Food Sci. 2018, 19, 1–7. [Google Scholar] [CrossRef]
- Oh, N.S.; Lee, J.Y.; Joung, J.Y.; Kim, K.S.; Shin, Y.K.; Lee, K.W.; Kim, S.H.; Oh, S.; Kim, Y. Microbiological Characterization and Functionality of Set-Type Yogurt Fermented with Potential Prebiotic Substrates Cudrania Tricuspidata and Morus Alba L. Leaf Extracts. J. Dairy Sci. 2016, 99, 6014–6025. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Gahruie, H.; Eskandari, M.H.; Mesbahi, G.; Hanifpour, M.A. Scientific and Technical Aspects of Yogurt Fortification: A Review. Food Sci. Hum. Wellness 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Carocho, M.; Oliveira, M.B.P.; Ferreira, I.C.F.R. Fortification of Yogurts with Different Antioxidant Preservatives: A Comparative Study between Natural and Synthetic Additives. Food Chem. 2016, 210, 262–268. [Google Scholar] [CrossRef]
- Mrabti, H.N.; Marmouzi, I.; Sayah, K.; Chemlal, L.; El Ouadi, Y.; Elmsellem, H.; Cherrah, H.; Faouzi, M.A. Arbutus unedo L. Aqueous Extract Is Associated with in Vitro and in Vivo Antioxidant Activity. J. Mater. Environ. Sci 2017, 8, 217–224. [Google Scholar]
- Iriondo-DeHond, M.; Blázquez-Duff, J.M.; del Castillo, M.D.; Miguel, E. Nutritional Quality, Sensory Analysis and Shelf Life Stability of Yogurts Containing Inulin-Type Fructans and Winery Byproducts for Sustainable Health. Foods 2020, 9, 1199. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Adney, B.; Baker, J. Measurement of Cellulase Activities: Laboratory Analytical Procedure (LAP); NERL/TP-510-42628; NREL: Golden, CO, USA, 1996. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oki, T.; Nagai, S.; Yoshinaga, M.; Nishiba, Y.; Suda, I. Contribution of B-Carotene to Radical Scavenging Capacity Varies among Orange-Fleshed Sweet Potato Cultivars. Food Sci. Technol. Res. 2006, 12, 156–160. [Google Scholar] [CrossRef]
- Dávalos, A.; Bartolomé, B.; Gómez-Cordovés, C. Antioxidant Properties of Commercial Grape Juices and Vinegars. Food Chem. 2005, 93, 325–330. [Google Scholar] [CrossRef]
- Afiukwa, C.; Ibiam, U.; Edeogu, C.; Nweke, F.; Chukwu, U. Determination of Amylase Activity of Crude Extract from Partially Germinated Mango Seeds (Mangifera Oraphila) T. Afr. J. Biotechnol. 2009, 8, 3294–3296. [Google Scholar]
- Zengin, G. A Study on in Vitro Enzyme Inhibitory Properties of Asphodeline Anatolica: New Sources of Natural Inhibitors for Public Health Problems. Ind. Crops Prod. 2016, 83, 39–43. [Google Scholar] [CrossRef]
- Herrera, T.; Navarro del Hierro, J.; Fornari, T.; Reglero, G.; Martin, D. Inhibitory Effect of Quinoa and Fenugreek Extracts on Pancreatic Lipase and α-Amylase under in Vitro Traditional Conditions or Intestinal Simulated Conditions. Food Chem. 2019, 270, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Geddes, R.; Taylor, J.A. Lysosomal Glycogen Storage Induced by Acarbose, a 1,4-Alpha-Glucosidase Inhibitor. Biochem. J. 1985, 228, 319–324. [Google Scholar] [CrossRef]
- Berthelot, K.; Delmotte, F.M. Purification and Characterization of an Alpha-Glucosidase from Rhizobium sp. (Robinia pseudoacacia L.) Strain USDA 4280. Appl. Environ. Microbiol. 1999, 65, 2907–2911. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Hochkogler, C.; Somoza, V.; del Castillo, M. Biscuits with No Added Sugar Containing Stevia, Coffee Fibre and Fructooligosaccharides Modifies α-Glucosidase Activity and the Release of GLP-1 from HuTu-80 Cells and Serotonin from Caco-2 Cells after In Vitro Digestion. Nutrients 2017, 9, 694. [Google Scholar] [CrossRef]
- Sugiyama, H.; Akazome, Y.; Shoji, T.; Yamaguchi, A.; Yasue, M.; Kanda, T.; Ohtake, Y. Oligomeric Procyanidins in Apple Polyphenol Are Main Active Components for Inhibition of Pancreatic Lipase and Triglyceride Absorption. J. Agric. Food Chem. 2007, 55, 4604–4609. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Intrawangso, J.; Hemrid, A.; Chanathong, B.; Mäkynen, K. Extracts of Edible Plants Inhibit Pancreatic Lipase, Cholesterol Esterase and Cholesterol Micellization, and Bind Bile Acids. Food Technol. Biotechnol. 2012, 50, 11–16. [Google Scholar]
- Vermeirssen, V.; Van Camp, J.; Verstraete, W. Optimisation and Validation of an Angiotensin-Converting Enzyme Inhibition Assay for the Screening of Bioactive Peptides. J. Biochem. Biophys. Methods 2002, 51, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, S.M.; Zakora, M.; Otte, J. Performance of Two Commonly Used Angiotensin-Converting Enzyme Inhibition Assays Using FA-PGG and HHL as Substrates. J. Dairy Res. 2006, 73, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Karnopp, A.R.; Oliveira, K.G.; de Andrade, E.F.; Postingher, B.M.; Granato, D. Optimization of an Organic Yogurt Based on Sensorial, Nutritional, and Functional Perspectives. Food Chem. 2017, 233, 401–411. [Google Scholar] [CrossRef] [PubMed]
- de Huidobro, F.R.; Miguel, E.; Blázquez, B.; Onega, E. A Comparison between Two Methods (Warner–Bratzler and Texture Profile Analysis) for Testing Either Raw Meat or Cooked Meat. Meat Sci. 2005, 69, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.; Ranocchia, K.; Marques, M.; da Silva, M.G.; Alves, V.; Coelhoso, I.; Simões, P. Evaluation of the Quality of Coffee Extracts Concentrated by Osmotic Evaporation. J. Food Eng. 2018, 222, 178–184. [Google Scholar] [CrossRef]
- Alirezalu, A.; Ahmadi, N.; Salehi, P.; Sonboli, A.; Alirezalu, K.; Khaneghah, A.M.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M. Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus spp.) Fruits Species for Potential Use in Food Applications. Foods 2020, 9, 436. [Google Scholar] [CrossRef]
- Fonseca, D.F.S.; Salvador, Â.C.; Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Silvestre, A.J.D.; Rocha, S.M. Bioactive Phytochemicals from Wild Arbutus unedo L. Berries from Different Locations in Portugal: Quantification of Lipophilic Components. Int. J. Mol. Sci. 2015, 16, 14194–14209. [Google Scholar] [CrossRef]
- Bebek Markovinović, A.; Brčić Karačonji, I.; Jurica, K.; Lasić, D.; Skendrović Babojelić, M.; Duralija, B.; Šic Žlabur, J.; Putnik, P.; Bursać Kovačević, D. Strawberry Tree Fruits and Leaves (Arbutus unedo L.) as Raw Material for Sustainable Functional Food Processing: A Review. Horticulturae 2022, 8, 881. [Google Scholar] [CrossRef]
- Vidrih, R.; Hribar, J.; Prgomet, Ž.; Ulrih, N.P. The Physico-Chemical Properties of Strawberry Tree (Arbutus unedo L.) Fruits. Croat. J. Food Sci. Technol. 2013, 5, 29–33. [Google Scholar]
- Colak, A.M. Morphological and Biochemical Diversity in Fruits of Arbutus unedo L. from East Aegean Region in Turkey. Erwerbs-Obstbau 2019, 61, 379–383. [Google Scholar] [CrossRef]
- Horžić, D.; Komes, D.; Belščak, A.; Ganić, K.K.; Iveković, D.; Karlović, D. The Composition of Polyphenols and Methylxanthines in Teas and Herbal Infusions. Food Chem. 2009, 115, 441–448. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, X.; Zhao, F.; Hou, G.; Meng, Q. Food Applications and Potential Health Benefits of Hawthorn. Foods 2022, 11, 2861. [Google Scholar] [CrossRef] [PubMed]
- Bnouham, M.; Ziyyat, A.; Mekhfi, H.; Tahri, A.; Legssyer, A. Medicinal Plants with Potential Antidiabetic Activity–A Review of Ten Years of Herbal Medicine Research (1990–2000). Int. J. Diabetes Metab. 2006, 14, 1–25. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Masteikova, R.; Majiene, D.; Savickas, A.; Kevelaitis, E.; Bernatoniene, R.; Dvořáčkovâ, K.; Civinskiene, G.; Lekas, R.; Vitkevičius, K.; et al. Free Radical-Scavenging Activities of Crataegus Monogyna Extracts. Medicina 2008, 44, 706. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.; Kucukislamoglu, M.; Reunanen, M. Sugar, Non-Volatile and Phenolic Acids Composition of Strawberry Tree (Arbutus unedo L. Var. Ellipsoidea) Fruits. J. Food Compos. Anal. 2000, 13, 171–177. [Google Scholar] [CrossRef]
- Mendes, L.; de Freitas, V.; Baptista, P.; Carvalho, M. Comparative Antihemolytic and Radical Scavenging Activities of Strawberry Tree (Arbutus unedo L.) Leaf and Fruit. Food Chem. Toxicol. 2011, 49, 2285–2291. [Google Scholar] [CrossRef]
- Zitouni, H.; Hssaini, L.; Ouaabou, R.; Viuda-Martos, M.; Hernández, F.; Ercisli, S.; Messaoudi, Z.; Hanine, H. Exploring Antioxidant Activity, Organic Acid, and Phenolic Composition in Strawberry Tree Fruits (Arbutus unedo L.) Growing in Morocco. Plants 2020, 9, 1677. [Google Scholar] [CrossRef]
- Pallauf, K.; Rivas-Gonzalo, J.C.; Del Castillo, M.D.; Cano, M.P.; De Pascual-Teresa, S. Characterization of the Antioxidant Composition of Strawberry Tree (Arbutus unedo L.) Fruits. J. Food Compos. Anal. 2008, 21, 273–281. [Google Scholar] [CrossRef]
- Yang, B.; Liu, P. Composition and Health Effects of Phenolic Compounds in Hawthorn (Crataegus spp.) of Different Origins. J. Sci. Food Agric. 2012, 92, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Svedström, U.; Vuorela, H.; Kostiainen, R.; Huovinen, K.; Laakso, I.; Hiltunen, R. High-Performance Liquid Chromatographic Determination of Oligomeric Procyanidins from Dimers up to the Hexamer in Hawthorn. J. Chromatogr. A 2002, 968, 53–60. [Google Scholar] [CrossRef]
- Ganhão, R.; Morcuende, D.; Estévez, M. Protein Oxidation in Emulsified Cooked Burger Patties with Added Fruit Extracts: Influence on Colour and Texture Deterioration during Chill Storage. Meat Sci. 2010, 85, 402–409. [Google Scholar] [CrossRef]
- Jakobek, L.; Barron, A.R. Ancient Apple Varieties from Croatia as a Source of Bioactive Polyphenolic Compounds. J. Food Compos. Anal. 2016, 45, 9–15. [Google Scholar] [CrossRef]
- Ceymann, M.; Arrigoni, E.; Schärer, H.; Bozzi Nising, A.; Hurrell, R.F. Identification of Apples Rich in Health-Promoting Flavan-3-Ols and Phenolic Acids by Measuring the Polyphenol Profile. J. Food Compos. Anal. 2012, 26, 128–135. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R. Optimization of a New Mobile Phase to Know the Complex and Real Polyphenolic Composition: Towards a Total Phenolic Index Using High-Performance Liquid Chromatography. J. Chromatogr. A 2003, 1018, 29–40. [Google Scholar] [CrossRef]
- Mišan, A.Č.; Mimica-Dukić, N.M.; Mandić, A.I.; Sakač, M.B.; Milovanović, I.L.; Sedej, I.J. Development of a Rapid Resolution HPLC Method for the Separation and Determination of 17 Phenolic Compounds in Crude Plant Extracts. Cent. Eur. J. Chem. 2011, 9, 133–142. [Google Scholar] [CrossRef]
- Wong, S.P.; Leong, L.P.; William Koh, J.H. Antioxidant Activities of Aqueous Extracts of Selected Plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Şeker, M.; Toplu, C. Determination and Comparison of Chemical Characteristics of Arbutus unedo L. and Arbutus andrachnae L. (Family Ericaceae) Fruits. J. Med. Food 2010, 13, 1013–1018. [Google Scholar] [CrossRef]
- Şahin, S.; Elhussein, E.; Bilgin, M.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S. Effect of Drying Method on Oleuropein, Total Phenolic Content, Flavonoid Content, and Antioxidant Activity of Olive (Olea europaea) Leaf. J. Food Process. Preserv. 2018, 42, e13604. [Google Scholar] [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Paul Ross, R. The α-Amylase and α-Glucosidase Inhibitory Effects of Irish Seaweed Extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Karaayak, P.E.; El, S.N.; Ercan, P. Inhibitory Effects of Chickpea and Tribulus Terrestris on Lipase, α-Amylase and α-Glucosidase. Food Chem. 2016, 205, 163–169. [Google Scholar] [CrossRef]
- Nunes, R.J.D.S. Micromedronho: Design of Microencapsulated Arbutus unedo Leaves and Fruits by Spray Drying for Supplements and Functional Foods; 2017. Available online: http://hdl.handle.net/10400.1/10796 (accessed on 27 August 2023).
- Matsui, T.; Tanaka, T.; Tamura, S.; Toshima, A.; Tamaya, K.; Miyata, Y.; Tanaka, K.; Matsumoto, K. α-Glucosidase Inhibitory Profile of Catechins and Theaflavins. J. Agric. Food Chem. 2007, 55, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Marathe, P.H.; Gao, H.X.; Close, K.L. American Diabetes Association Standards of Medical Care in Diabetes 2017. J. Diabetes 2017, 9, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Mrabti, H.N.; Jaradat, N.; Fichtali, I.; Ouedrhiri, W.; Jodeh, S.; Ayesh, S.; Cherrah, Y.; Faouzi, M.E.A. Separation, Identification, and Antidiabetic Activity of Catechin Isolated from Arbutus unedo L. Plant Roots. Plants 2018, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J.; Yin, M.C. Antioxidative and Anti-Inflammatory Protection of Oleanolic Acid and Ursolic Acid in PC12 Cells. J. Food Sci. 2008, 73, 174–178. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.-H. Anti-Inflammatory Effect of the Water Fraction from Hawthorn Fruit on LPS-Stimulated RAW 264.7 Cells. Nutr. Res. Pract. 2011, 5, 101–106. [Google Scholar] [CrossRef]
- Keser, S.; Celik, S.; Turkoglu, S.; Yilmaz, Ö.; Turkoglu, I. Hydrogen Peroxide Radical Scavenging and Total Antioxidant Activity of Hawthorn. Chem. J. 2012, 2, 9–12. [Google Scholar]
- Miao, J.; Zhao, C.; Li, X.; Chen, X.; Mao, X.; Huang, H.; Wang, T.; Gao, W. Chemical Composition and Bioactivities of Two Common Chaenomeles Fruits in China: Chaenomeles Speciosa and Chaenomeles Sinensis. J. Food Sci. 2016, 81, H2049–H2058. [Google Scholar] [CrossRef]
- Zhao, C.; Miao, J.; Li, X.; Chen, X.; Mao, X.; Wang, Y.; Hua, X.; Gao, W. Impact of in Vitro Simulated Digestion on the Chemical Composition and Potential Health Benefits of Chaenomeles speciosa and Crataegus pinnatifida. Food Biosci. 2020, 35, 100511. [Google Scholar] [CrossRef]
- Miao, J.; Li, X.; Fan, Y.; Zhao, C.; Mao, X.; Chen, X.; Huang, H.; Gao, W. Effect of Different Solvents on the Chemical Composition, Antioxidant Activity and Alpha-Glucosidase Inhibitory Activity of Hawthorn Extracts. Int. J. Food Sci. Technol. 2016, 51, 1244–1251. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-Amylase, α-Glucosidase and Lipase Inhibitory Activity of the Phenolic Substances in Two Black Legumes of Different Genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, P.M.; Sreerama, Y.N. Phenolic Antioxidants of Foxtail and Little Millet Cultivars and Their Inhibitory Effects on α-Amylase and α-Glucosidase Activities. Food Chem. 2018, 247, 46–55. [Google Scholar] [CrossRef]
- Isbilir, S.S.; Kabala, S.I.; Yagar, H. Assessment of in Vitro Antioxidant and Antidiabetic Capacities of Medlar (Mespilus germanica). Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 384–389. [Google Scholar] [CrossRef]
- Birari, R.B.; Bhutani, K.K. Pancreatic Lipase Inhibitors from Natural Sources: Unexplored Potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef]
- Buchholz, T.; Chen, C.; Zhang, X.; Melzig, M.F. Pancreatic Lipase and α-Amylase Inhibitory Activities of Plants Used in Traditional Chinese Medicine (TCM). Pharmazie 2016, 71, 420–424. [Google Scholar] [CrossRef]
- Seyedan, A.; Alshawsh, M.A.; Alshagga, M.A.; Koosha, S.; Mohamed, Z. Medicinal Plants and Their Inhibitory Activities against Pancreatic Lipase: A Review. Evid. Based Complement. Altern. Med. 2015, 2015, 973143. [Google Scholar] [CrossRef]
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G.A. Effects of Saponins on Lipid Metabolism: A Review of Potential Health Benefits in the Treatment of Obesity. Molecules 2016, 21, 1404. [Google Scholar] [CrossRef]
- Mollica, A.; Zengin, G.; Locatelli, M.; Stefanucci, A.; Mocan, A.; Macedonio, G.; Carradori, S.; Onaolapo, O.; Onaolapo, A.; Adegoke, J.; et al. Anti-Diabetic and Anti-Hyperlipidemic Properties of Capparis Spinosa L.: In Vivo and in Vitro Evaluation of Its Nutraceutical Potential. J. Funct. Foods 2017, 35, 32–42. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Zengin, G.; Segura-Carretero, A.; Lobine, D.; Mahomoodally, M.F. Chemical Fingerprint and Bioactivity Evaluation of Globularia orientalis L. and Globularia trichosantha Fisch. & C. A. Mey. Using Non-Targeted HPLC-ESI-QTOF-MS Approach. Phytochem. Anal. 2019, 30, 237–252. [Google Scholar] [CrossRef]
- Aierken, A.; Buchholz, T.; Chen, C.; Zhang, X.; Melzig, M.F. Hypoglycemic Effect of Hawthorn in Type II Diabetes Mellitus Rat Model. J. Sci. Food Agric. 2017, 97, 4557–4561. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, F.; Xing, J.; Tsao, R.; Liu, Z.; Liu, S. Screening and Structural Characterization of α-Glucosidase Inhibitors from Hawthorn Leaf Flavonoids Extract by Ultrafiltration LC-DAD-MSn and SORI-CID FTICR MS. J. Am. Soc. Mass Spectrom. 2009, 20, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.F.; Marakis, G.; Morris, A.P.; Robinson, P.A. Promising Hypotensive Effect of Hawthorn Extract: A Randomized Double-Blind Pilot Study of Mild, Essential Hypertension. Phyther. Res. 2002, 16, 48–54. [Google Scholar] [CrossRef]
- Lacaille-Dubois, M.A.; Franck, U.; Wagner, H. Search for Potential Angiotensin Converting Enzyme (ACE)-Inhibitors from Plants. Phytomedicine 2001, 8, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, C.V. José Rodríguez-Vaquero Berries as Source of Bioactive Compounds. Emerg. Environ. Technol. Health Prot. 2019, 2, 28–36. [Google Scholar]
- González, E.A.; Agrasar, A.T.; Castro, L.M.P.; Fernández, I.O.; Guerra, N.P. Solid-State Fermentation of Red Raspberry (Rubus ideaus L.) and Arbutus Berry (Arbutus unedo L.) and Characterization of Their Distillates. Food Res. Int. 2011, 44, 1419–1426. [Google Scholar] [CrossRef]
- Kebal, L.; Pokajewicz, K.; Djebli, N.; Mostefa, N.; Poliwoda, A.; Wieczorek, P.P. HPLC-DAD Profile of Phenolic Compounds and In vitro Antioxidant Activity of Ficus carica L. Fruits from Two Algerian Varieties. Biomed. Pharmacother. 2022, 155, 113738. [Google Scholar] [CrossRef] [PubMed]
- Masmoudi, M.; Ammar, I.; Ghribi, H.; Attia, H. Physicochemical, Radical Scavenging Activity and Sensory Properties of a Soft Cheese Fortified with Arbutus unedo L. Extract. Food Biosci. 2020, 35, 100579. [Google Scholar] [CrossRef]
- Oliveira, A.; Alexandre, E.M.C.; Coelho, M.; Lopes, C.; Almeida, D.P.F.; Pintado, M. Incorporation of Strawberries Preparation in Yoghurt: Impact on Phytochemicals and Milk Proteins. Food Chem. 2015, 171, 370–378. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. The Definition and Measurement of Antioxidants in Biological Systems. Free Radic. Biol. Med. 1995, 18, 125–126. [Google Scholar] [CrossRef]
- Armenteros, M.; Morcuende, D.; Ventanas, S.; Estévez, M. Application of Natural Antioxidants from Strawberry Tree (Arbutus unedo L.) and Dog Rose (Rosa canina L.) to Frankfurters Subjected to Refrigerated Storage. J. Integr. Agric. 2013, 12, 1972–1981. [Google Scholar] [CrossRef]
- Ganhao, R.U.I.; Estévez, M.; Armenteros, M.; Morcuende, D. Mediterranean Berries as Inhibitors of Lipid Oxidation in Porcine Burger Patties Subjected to Cooking and Chilled Storage. J. Integr. Agric. 2013, 12, 1982–1992. [Google Scholar] [CrossRef]
- Cossu, M.; Juliano, C.; Pisu, R.; Alamanni, M.C. Effects of Enrichment with Polyphenolic Extracts from Sardinian Plants on Physico-Chemical, Antioxidant and Microbiological Properties of Yogurt. Ital. J. Food Sci. 2009, 21, 447–459. [Google Scholar]
- Cereceres Aragón, A.; Rodríguez Tadeo, A.; Álvarez Parrilla, E.; Rodrigo García, J. Ingestión de Compuestos Fenólicos En Población Adulta Mayor. Nutr. Hosp. 2018, 36, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Kahriman, N.; Albay, C.; Usta, A.; Karaoğlu, Ş.A.; Yayli, N. Volatile Constituents and Antimicrobial Activities from Flower and Fruit of Arbutus unedo L. Asian J. Chem. 2010, 22, 6437–6442. [Google Scholar]
- Hong, H.; Lim, J.M.; Kothari, D.; Kwon, S.H.; Kwon, H.C.; Han, S.-G.; Kim, S.-K. Antioxidant Properties and Diet-Related α-Glucosidase and Lipase Inhibitory Activities of Yogurt Supplemented with Safflower (Carthamus Tinctorius L.) Petal Extract. Food Sci. Anim. Resour. 2021, 41, 122–134. [Google Scholar] [CrossRef]
- Donkor, O.N.; Henriksson, A.; Singh, T.K.; Vasiljevic, T.; Shah, N.P. ACE-Inhibitory Activity of Probiotic Yoghurt. Int. Dairy J. 2007, 17, 1321–1331. [Google Scholar] [CrossRef]
- Amirdivani, S.; Baba, A.S. Changes in Yogurt Fermentation Characteristics, and Antioxidant Potential and in Vitro Inhibition of Angiotensin-1 Converting Enzyme upon the Inclusion of Peppermint, Dill and Basil. LWT Food Sci. Technol. 2011, 44, 1458–1464. [Google Scholar] [CrossRef]
- Pelaes Vital, A.C.; Goto, P.A.; Hanai, L.N.; Gomes-da-Costa, S.M.; de Abreu Filho, B.A.; Nakamura, C.V.; Matumoto-Pintro, P.T. Microbiological, Functional and Rheological Properties of Low Fat Yogurt Supplemented with Pleurotus ostreatus Aqueous Extract. LWT Food Sci. Technol. 2015, 64, 1028–1035. [Google Scholar] [CrossRef]
- Do Espírito Santo, A.P.; Cartolano, N.S.; Silva, T.F.; Soares, F.A.S.M.; Gioielli, L.A.; Perego, P.; Converti, A.; Oliveira, M.N. Fibers from Fruit By-Products Enhance Probiotic Viability and Fatty Acid Profile and Increase CLA Content in Yoghurts. Int. J. Food Microbiol. 2012, 154, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, T.C.; Cruz, A.G.; Prudencio, S.H. Short Communication: Influence of Long-Chain Inulin and Lactobacillus paracasei Subspecies Paracasei on the Sensory Profile and Acceptance of a Traditional Yogurt. J. Dairy Sci. 2013, 96, 6233–6241. [Google Scholar] [CrossRef] [PubMed]
- Atamian, S.; Olabi, A.; Kebbe Baghdadi, O.; Toufeili, I. The Characterization of the Physicochemical and Sensory Properties of Full-Fat, Reduced-Fat and Low-Fat Bovine, Caprine, and Ovine Greek Yogurt (Labneh). Food Sci. Nutr. 2014, 2, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Cliff, M.A.; Fan, L.; Sanford, K.; Stanich, K.; Doucette, C.; Raymond, N. Descriptive Analysis and Early-Stage Consumer Acceptance of Yogurts Fermented with Carrot Juice. J. Dairy Sci. 2013, 96, 4160–4172. [Google Scholar] [CrossRef]
- Drub, T.; dos Santos, F.; Centeno, A.; Capriles, V. Sorghum, Millet and Pseudocereals as Ingredients for Gluten-Free Whole-Grain Yeast Rolls. Int. J. Gastron. Food Sci. 2021, 23, 100293. [Google Scholar] [CrossRef]
- Torrico, D.D.; Tam, J.; Fuentes, S.; Viejo, C.G.; Dunshea, F.R. D-Tagatose as a Sucrose Substitute and Its Effect on the Physico-Chemical Properties and Acceptability of Strawberry-Flavored Yogurt. Foods 2019, 8, 256. [Google Scholar] [CrossRef]
- Wajs, J.; Brodziak, A.; Król, J. Shaping the Physicochemical, Functional, Microbiological and Sensory Properties of Yoghurts Using Plant Additives. Foods 2023, 12, 1275. [Google Scholar] [CrossRef]


| Chemical | STE | HE |
|---|---|---|
| Proteins (g/100 g extract) | 1.40 ± 0.06 a | 2.62 ± 0.10 b |
| Reducing sugar (g/100 g extract) | 48.50 ± 5.10 b | 33.24 ± 8.62 a |
| TPC (mg GAE/g extract) | 17.93 ± 4.17 a | 22.01 ± 1.16 a |
| Antioxidant capacity | ||
| ABTS (µmol TE/g extract) | 573.24 ± 10.31 a | 731.00 ± 32.29 a |
| ORAC (µmol TE/g extract) | 190.39 ± 7.35 a | 625.94 ± 27.60 b |
| Antidiabetic properties | ||
| α-Amylase inhibition (%) | 5.46 ± 0.65 a | 6.02 ± 0.44 a |
| α-Glucosidase inhibition (%) | 54.07 ± 9.90 a | 57.92 ± 0.92 b |
| α-Glucosidase IC50 (mg/mL) | 7.26 ± 0.34 a | 8.01 ± 0.27 b |
| Lipase inhibition (%) | 97.79 ± 15.74 a | 91.01 ± 2.88 a |
| Lipase IC50 (mg/mL) | 8.14 ± 0.50 b | 3.63 ± 0.37 a |
| Antihypertensive properties | ||
| ECA inhibition (%) | 20.71 ± 1.83 b | 14.27 ± 1.03 a |
| Compounds | TR | M/Z [M-H] | Formula | STE | HE |
|---|---|---|---|---|---|
| Catechin | 9.8 | 289.0718 | C15H14O6 | 0.40 ± 0.03 a | 0.19 ± 0.03 b |
| Epicatechin | 14.4 | 289.0718 | C15H14O6 | 0.01 ± 0.00 a | 8.12 ± 1.03 b |
| Quercetin Galactoside | 23.7 | 463.0882 | C21H20O12 | n.d. | 2.22 ± 0.34 |
| Quercetin Glucoside | 24.4 | 463.0882 | C21H20O12 | n.d. | 1.36 ± 0.19 |
| Quercetin Glucoside Acetate | 25.8 | 505.0988 | C23H22O13 | n.d. | 0.14 ± 0.02 |
| Procyanidin Dimer (I) | 8.2 | 577.1351 | C30H26O12 | 0.10 ± 0.01 | n.d. |
| Procyanidin Dimer (II) | 8.9 | 577.1351 | C30H26O12 | 0.07 ± 0.01 | n.d. |
| Procyanidin Dimer (III) | 12.7 | 577.1351 | C30H26O12 | n.d. | 4.34 ± 0.88 |
| Procyanidin Dimer (IV) | 14.3 | 577.1351 | C30H26O12 | 0.01 ± 0.00 | n.d. |
| Procyanidin Dimer (V) | 17.9 | 577.1351 | C30H26O12 | 0.01 ± 0.00 | n.d. |
| Quercetin Rutinoside | 23.2 | 609.1461 | C27H30O16 | n.d. | 0.25 ± 0.88 |
| Quercetin Diglucoside | 19 | 625.141 | C27H30O17 | n.d. | 0.09 ± 0.01 |
| Procyanidin Trimer | 16.81 | 865.1985 | C45H38O18 | n.d. | 1.52 ± 0.38 |
| TOTAL Flavonoids | 0.59 ± 0.06 a | 18.38 ± 2.37 b | |||
| Fertaric Acid (I) | 4.6 | 325.0565 | C14H14O9 | 0.07 ± 0.01 | n.d. |
| Hydroxybenzyl Tartaric Acid | 4.8 | 255.051 | C11H12O7 | n.d. | 1.15 ± 0.16 |
| Fertaric Acid (II) | 5.1 | 325.0565 | C14H14O9 | 0.25 ± 0.01 | n.d. |
| Galloyl Glucose | 4.3 | 331.0671 | C13H16O10 | 0.40 ± 0.10 | n.d. |
| Coumaroylquinic Acid | 9.1 | 337.0929 | C16H18O8 | n.d. | 0.22 ± 0.02 |
| Theogallin | 2.8 | 343.0671 | C14H16O10 | 1.89 ± 0.38 | n.d. |
| Caffeoylquinic Acid (I) | 6.4 | 353.0887 | C16H18O9 | n.d. | 0.32 ± 0.06 |
| Caffeoylquinic Acid (II) | 10.3 | 353.0887 | C16H18O9 | n.d. | 0.70 ± 0.12 |
| Caffeoylquinic Acid (III) | 11.1 | 353.0887 | C16H18O9 | n.d. | 0.12 ± 0.02 |
| Caffeoylquinic Acid (IV) | 13.9 | 353.0887 | C16H18O9 | n.d. | 0.05 ± 0.00 |
| Ellagic Acid Arabinoside | 22 | 433.0412 | C19H14O12 | 0.13 ± 0.05 | n.d. |
| Ellagic Acid Glucoside | 17.1 | 463.0518 | C20H16O13 | 0.11 ± 0.04 | n.d. |
| DigalloylShikimic Acid | 12.9 | 477.0675 | C21H18O13 | 0.43 ± 0.21 | n.d. |
| Theogallin Derivative | 9.2 | 495.0780 | C21H20O14 | 0.69 ± 0.37 | n.d. |
| Strictinin | 21.2 | 581.2240 | C28H38O13 | 0.06 ± 0.01 | n.d. |
| TOTAL Phenolics acids | 3.98 ± 0.82 b | 2.57 ± 0.27 a | |||
| Cyanidin Glucoside | 11.6 | 447.0933 | C21H21O11 | 0.11 ± 0.01 a | 1.30 ± 0.13 b |
| Cyanidin Arabinoside | 13.7 | 419.0973 | C20H19O10 | 0.06 ± 0.00 | n.d. |
| TOTAL Anthocyanins | 0.17 ± 0.01 a | 1.30 ± 0.13 b | |||
| Total Phenolic Compounds | 4.74 ± 0.87 a | 22.25 ± 2.77 b | |||
| Strawberry Tree Yogurts | Hawthorn Yogurts | ||||
|---|---|---|---|---|---|
 Control |  YSTE-8 |  YSTE-12 |  YHE-8 |  YHE-12 | |
| Proteins (%) | 1.49 ± 0.01 a | 2.10 ± 0.04 b | 2.07 ± 0.19 b | 1.99 ± 0.07 b | 2.10 ± 0.01 b |
| Reducing sugar (%) | 3.36 ± 0.04 a | 3.50 ± 0.15 a | 3.55 ± 0.26 a | 3.11 ± 0.00 a | 3.25 ± 0.00 a |
| Lactose (%) | 9.67 ± 0.42 a | 10.13 ± 0.70 a | 10.40 ± 0.35 a | 5.67 ± 0.3 a | 6.13 ± 0.31 a |
| TPC (mg GAE/g yogurt) | 0.07 ± 0.02 a | 0.11 ± 0.04 a | 0.10 ± 0.04 a | 0.12 ± 0.05 a | 0.17 ± 0.09 a |
| Antioxidant capacity | |||||
| ABTS (µmol TE/g extract) | 0.59 ± 0.25 a | 0.85 ± 0.22 a | 0.89 ± 0.27 a | 0.87 ± 0.24 a | 0.96 ± 0.26 a |
| ORAC (µmol TE/g extract) | 0.58 ± 0.09 a | 2.98 ± 0.36 a | 2.69 ± 0.32 a | 8.61 ± 0.70 b | 15.82 ± 2.56 c |
| Antidiabetic properties | |||||
| α-Amylase inhibition (%) | 2.79 ± 0.23 a | 3.10 ± 0.48 ab | 3.53 ± 0.45 b | 3.29 ± 0.15 b | 3.84 ± 0.07 b |
| α-Glucosidase inhibition (%) | 9.96 ± 0.86 a | 23.84 ± 6.34 ab | 27.55 ± 3.88 b | 22.69 ± 5.09 ab | 23.09 ± 4.92 ab |
| Lipase inhibition (%) | 44.14 ± 6.44 a | 49.48 ± 2.39 a | 50.86 ± 4.00 a | 45.38 ± 2.22 a | 46.17 ± 5.35 a |
| Antihypertensive properties | |||||
| ACE inhibition (%) | 16.37 ± 2.67 a | 15.65 ± 1.40 a | 15.69 ± 0.80 a | 16.50 ± 2.79 a | 17.49 ± 1.24 a |
| Physicochemical parameters | |||||
| pH | 4.50 ± 0.06 a | 4.49 ± 0.09 a | 4.50 ± 0.02 a | 4.57 ± 0.04 a | 4.51 ± 0.07 a |
| Moisture (%) | 83.60 ± 1.97 a | 84.04 ± 1.55 a | 83.04 ± 0.51 a | 83.81 ± 1.12 a | 83.62 ± 0.40 a |
| Syneresis (%) | 50.67 ± 3.92 a | 48.77 ± 7.99 a | 53.19 ± 4.94 a | 55.54 ± 0.84 a | 51.98 ± 2.69 a |
| Viscosity (Pas) | 3.70 ± 0.39 a | 3.53 ± 0.89 a | 3.35 ± 0.96 a | 3.01 ± 0.26 a | 3.40 ± 0.72 a |
| Compounds | Strawberry Tree Yogurts | Hawthorn Yogurts | |||
|---|---|---|---|---|---|
| YSTE-8 | YSTE-12 | YHE-8 | YHE-12 | ||
| Flavonoids | Catechin | 0.63 ± 0.17 b | 0.73 ± 0.08 b | 0.16 ± 0.02 a | 0.16 ± 0.02 a |
| Epicatechin | 0.03 ± 0.00 a | 0.03 ± 0.01 a | 21.96 ± 0.72 b | 35.35 ± 11.99 b | |
| Quercetin Galactoside | n.d. | n.d. | 1.70 ± 0.27 | 3.29 ± 0.59 | |
| Quercetin Glucoside | n.d. | n.d. | 0.82 ± 0.03 | 1.76 ± 0.69 | |
| Quercetin Glucoside Acetate | n.d. | n.d. | 0.02 ± 0.00 | 0.04 ± 0.00 | |
| Procyanidin Dimer (I) | 0.05 ± 0.02 | 0.06 ± 0.00 | n.d. | n.d. | |
| Procyanidin Dimer (II) | 0.05 ± 0.01 | 0.08 ± 0.00 | n.d. | n.d. | |
| Procyanidin Dimer (II) | n.d. | n.d. | 6.80 ± 0.41 | 9.89 ± 8.56 | |
| Procyanidin Dimer (III) | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | |
| Procyanidin Dimer (IV) | 0.00 ± 0.00 a | 0.00 ± 0.00 a | n.d. | n.d. | |
| Quercetin Rutinoside | n.d. | n.d. | 0.33 ± 0.01 | 0.56 ± 0.13 | |
| Quercetin Diglucoside | n.d. | n.d. | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Procyanidin Trimer | n.d. | n.d. | 0.15 ± 0.04 | 0.33 ± 0.01 | |
| TOTALFlavonoids | 0.76 ± 0.21 a | 0.91 ± 0.10 a | 32.09 ± 1.47 b | 51.47 ± 22.58 b | |
| Phenolics acids | Hydroxybenzyl Tartaric Acid | n.d. | n.d. | 3.68 ± 0.07 | 8.81 ± 0.24 |
| Fertaric Acid (I) | 0.34 ± 0.07 | 0.36 ± 0.06 | n.d. | n.d. | |
| Fertaric Acid (II) | 1.44 ± 0.31 | 1.57 ± 0.21 | n.d. | n.d. | |
| Galloyl Glucose | 1.38 ± 0.30 | 1.56 ± 0.20 | n.d. | n.d. | |
| Coumaroylquinic Acid | n.d. | n.d. | 0.85 ± 0.00 | 1.76 ± 0.06 | |
| Theogallin | 13.01 ± 1.97 a | 14.27 ± 0.99 a | n.d. | n.d. | |
| Caffeoylquinic Acid (I) | n.d. | n.d. | 1.82 ± 0.07 | 3.57 ± 0.76 | |
| Caffeoylquinic Acid (II) | n.d. | n.d. | 2.02 ± 0.04 | 3.57 ± 0.83 | |
| Caffeoylquinic Acid (III) | n.d. | n.d. | 0.64 ± 0.00 | 19.69 ± 0.28 | |
| Caffeoylquinic Acid (IV) | n.d. | n.d. | 0.41 ± 0.06 | 0.93 ± 0.16 | |
| Ellagic Acid Arabinoside | 0.05 ± 0.01 | 0.05 ± 0.02 | n.d. | n.d. | |
| Ellagic Acid Glucoside | 0.07 ± 0.01 | 0.08 ± 0.03 | n.d. | n.d. | |
| DigalloylShikimic Acid | 0.28 ± 0.14 | 0.29 ± 0.10 | n.d. | n.d. | |
| Theogallin Derivative | 0.63 ± 0.24 | 0.70 ± 0.24 | n.d. | n.d. | |
| Strictinin | 0.12 ± 0.03 | 0.19 ± 0.01 | n.d. | n.d. | |
| TOTALPhenolic sacids | 17.31 ± 3.06 ab | 19.07 ± 1.82 ab | 9.43 ± 0.02 a | 25.91 ± 2.33 c | |
| Anthocyanins | Cyanidin Glucoside | 0.27 ± 0.06 a | 0.34 ± 0.04 a | 3.81 ± 2.20 b | 3.33 ± 2.41 b |
| Cyanidin Arabinoside | 0.06 ± 0.01 | 0.07 ± 0.01 | n.d. | n.d. | |
| TOTALAnthocyanin | 0.33 ± 0.07 a | 0.41 ± 0.05 a | 3.81 ± 0.09 b | 3.33 ± 1.41 b | |
| Total Phenolic Compounds | 18.40 ± 3.28 a | 20.39 ± 1.96 a | 45.33 ± 21.78 b | 93.13 ± 26.99 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, T.; Iriondo-DeHond, M.; Ramos Sanz, A.; Bautista, A.I.; Miguel, E. Effect of Wild Strawberry Tree and Hawthorn Extracts Fortification on Functional, Physicochemical, Microbiological, and Sensory Properties of Yogurt. Foods 2023, 12, 3332. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12183332
Herrera T, Iriondo-DeHond M, Ramos Sanz A, Bautista AI, Miguel E. Effect of Wild Strawberry Tree and Hawthorn Extracts Fortification on Functional, Physicochemical, Microbiological, and Sensory Properties of Yogurt. Foods. 2023; 12(18):3332. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12183332
Chicago/Turabian StyleHerrera, Teresa, Maite Iriondo-DeHond, Ana Ramos Sanz, Ana Isabel Bautista, and Eugenio Miguel. 2023. "Effect of Wild Strawberry Tree and Hawthorn Extracts Fortification on Functional, Physicochemical, Microbiological, and Sensory Properties of Yogurt" Foods 12, no. 18: 3332. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12183332