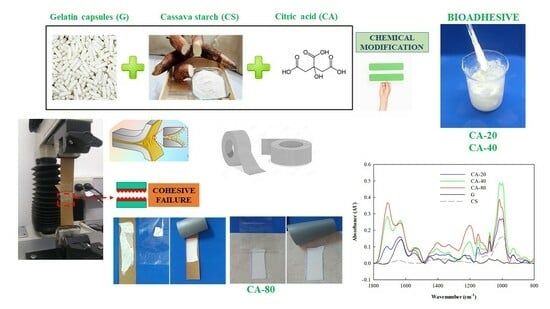
Liquid and Pressure-Sensitive Adhesives Based on Cassava Starch and Gelatin Capsule Residue: Green Alternatives for the Packaging Industry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formulation of Biobased Adhesives
2.1.1. Rheological Characterization
2.1.2. Back Extrusion
2.2. Adhesive Capacity of Liquid Formulations
2.3. Analysis of the Microstructure by ATR-FTIR and SEM
2.4. Contact Angle
2.5. Evaluation of the Adhesive Capacity of Pressure-Sensitive Formulation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Rheological Behavior of Composite Adhesive Formulations
3.2. Adhesive Capacity of Composite Formulations
3.3. Structural Modifications Induced in Adhesive Formulations: ATR-FTIR Studies
3.4. Pressure-Sensitive Adhesive: Characteristics and Potential Applications
4. Conclusions
5. Patent
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortega, F.; Versino, F.; López, O.V.; García, M.A. Biobased composites from agro-industrial wastes and by-products. Emergent Mater. 2022, 5, 873–921. [Google Scholar] [CrossRef]
- Dunky, M. Applications and Industrial Implementations of Naturally-Based Adhesives. In Biobased Adhesives: Sources, Characteristics and Applications; Scrivener Publishing LLC: Beverly, MA, USA, 2023; pp. 659–704. [Google Scholar]
- Rizky, S.; Wintoko, J. Modification of bioadhesive based on crosslinked alginate and gelatin. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Uranga, J.; Nguyen, B.T.; Si, T.T.; Guerrero, P.; de la Caba, K. The effect of cross-linking with citric acid on the properties of agar/fish gelatin films. Polymers 2020, 12, 291. [Google Scholar] [CrossRef] [PubMed]
- Vargas, C.G.; Mercali, G.D.; de Oliveira Rios, A.; Rahier, H.; Flôres, S.H. Effect of moderate electric field on the properties of gelatin capsule residue-based films. Food Hydrocoll. 2019, 89, 29–35. [Google Scholar]
- Versino, F.; Ortega, F.; Monroy, Y.; Rivero, S.; López, O.V.; García, M.A. Sustainable and bio-based food packaging: A review on past and current design innovations. Foods 2023, 12, 1057. [Google Scholar] [CrossRef]
- Wangtueai, S.; Chaiyaso, T.; Rachtanapun, P.; Jantrawut, P.; Ruksiriwanich, W.; Seesuriyachan, P.; Leksawasdi, N.; Phimolsiripol, Y.; Techapun, C.; Phongthai, S.; et al. Thermoplastic cassava starch blend with polyethylene-grafted-maleic anhydride and gelatin core-shell structure compatibilizer. Int. J. Biol. Macromol. 2022, 197, 49–54. [Google Scholar] [CrossRef]
- Luque, G.C.; Stürtz, R.; Passeggi Jr, M.C.; Gugliotta, L.M.; Gonzalez, V.D.; Minari, R.J. New hybrid acrylic/collagen nanocomposites and their potential use as bio-adhesives. Int. J. Adhes. Adhes. 2020, 100, 102624. [Google Scholar] [CrossRef]
- Costa TM, H.; de Oliveira Rios, A.; Flôres, S.H. Residues of minimally processed carrot and gelatin capsules: Potential materials for packaging films. Ind. Crops Prod. 2015, 76, 1071–1078. [Google Scholar]
- Iahnke, A.O.E.S.; Stoll, L.; Bellé, A.S.; Hertz, P.F.; Rios, A.D.O.; Rahier, H.; Flôres, S.H. Gelatin capsule residue-based films crosslinked with the natural agent genipin. Packag. Technol. Sci. 2020, 33, 15–26. [Google Scholar] [CrossRef]
- García-Campo, M.J.; Boronat, T.; Quiles-Carrillo, L.; Balart, R.; Montanes, N. Manufacturing and characterization of toughened poly (lactic acid)(PLA) formulations by ternary blends with biopolyesters. Polymers 2017, 10, 3. [Google Scholar] [CrossRef]
- Liguori, A.; Uranga, J.; Panzavolta, S.; Guerrero, P.; de la Caba, K.; Focarete, M.L. Electrospinning of fish gelatin solution containing citric acid: An environmentally friendly approach to prepare crosslinked gelatin fibers. Materials 2019, 12, 2808. [Google Scholar] [CrossRef] [PubMed]
- Monroy, Y. Desarrollo de Bioadhesivos a Base de Almidones Modificados Con Aplicaciones Potenciales en el Área de Envases. Doctoral Dissertation, Universidad Nacional de La Plata, La Plata, Argentina, 2021. [Google Scholar]
- Cohen, E.; Binshtok, O.; Dotan, A.; Dodiuk, H. Prospective materials for biodegradable and/or biobased pressure-sensitive adhesives: A review. J. Adhes. Sci. Technol. 2013, 27, 1998–2013. [Google Scholar] [CrossRef]
- Sartori, T.; Feltre, G.; do Amaral Sobral, P.J.; da Cunha, R.L.; Menegalli, F.C. Biodegradable pressure sensitive adhesives produced from vital wheat gluten: Effect of glycerol as plasticizer. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 42–49. [Google Scholar] [CrossRef]
- Monroy, Y.; Rivero, S.M.G.; García, M.A. Composiciones Adhesivas Ecocompatibles y Película Adhesiva Bifaz, y Procedimientos de Elaboración; Acta No. 20220102305; INPI: Buenos Aires, Argentina, 2022. [Google Scholar]
- Monroy, Y.; Cabezas, D.M.; Rivero, S.; García, M.A. Structural analysis and adhesive capacity of cassava starch modified with NaOH: Urea mixtures. Int. J. Adhes. Adhes. 2023, 126, 103470. [Google Scholar] [CrossRef]
- Monroy, Y.; Hamet, M.F.; Rivero, S.; García, M.A. Tailor-made starch-based adhesives chemically modified with NaOH: Urea and their applications on a cellulosic substrate. Int. J. Biol. Macromol. 2023, 247, 125423. [Google Scholar] [CrossRef]
- Monroy, Y.; Rivero, S.; García, M.A. Sustainable panels design based on modified cassava starch bioadhesives and wood processing byproducts. Ind. Crop. Prod. 2019, 137, 171–179. [Google Scholar] [CrossRef]
- ASTM Committee D-1 on Paint and Related Coatings, Materials, and Applications. In Standard Test Methods for Measuring Adhesion by Tape Test; ASTM International: West Conshohocken, PA, USA, 2009.
- Firoozmand, H.; Murray, B.S.; Dickinson, E. Microstructure elastic modulus of mixed gels of gelatin-oxidized starch: Effect of, p.H. Food Hydrocoll. 2012, 26, 286–292. [Google Scholar] [CrossRef]
- Goudie, K.J.; McCreath, S.J.; Parkinson, J.A.; Davidson, C.M.; Liggat, J.J. Investigation of the influence of pH on the properties and morphology of gelatin hydrogels. J. Polym. Sci. 2023, 61, 2316–2332. [Google Scholar] [CrossRef]
- Wang, R.; Hartel, R.W. Citric acid and heating on gelatin hydrolysis and gelation in confectionery gels. Food Hydrocoll. 2022, 129, 107642. [Google Scholar] [CrossRef]
- Fang, C.; Wu, C.; Zhao, X. Preparation and characterization of high solid content acrylate latex pressure sensitive adhesives with difunctional cross-linker EGDMA. Int. J. Adhes. Adhes. 2023, 125, 103403. [Google Scholar] [CrossRef]
- Kumar, R.; Ghoshal, G.; Goyal, M. Synthesis and functional properties of gelatin/CA–starch composite film: Excellent food packaging material. J. Food Sci. Technol. 2019, 56, 1954–1965. [Google Scholar] [CrossRef] [PubMed]
- Dohr, C.A.; Hirn, U. Influence of paper properties on adhesive strength of starch gluing. Nord. Pulp Pap. Res. J. 2022, 37, 120–129. [Google Scholar] [CrossRef]
- Ocaña López, R. Degradación Ambiental y en Condiciones Adversas de Adhesivos Estructurales: Análisis y Consideraciones técnicas Para su Aplicación Industrial. Doctoral Dissertation, Universidad Politécnica de Madrid, Madrid, España, 2017. [Google Scholar]
- He, Q.; Zhang, Y.; Cai, X.; Wang, S. Fabrication of gelatin–TiO2 nanocomposite film and its structural, antibacterial and physical properties. Int. J. Biol. Macromol. 2016, 84, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ghoshal, G.; Goyal, M. Biodegradable composite films/coatings of modified corn starch/gelatin for shelf life improvement of cucumber. J. Food Sci. Technol. 2020, 58, 1227–1237. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702. [Google Scholar] [CrossRef]
- Brandelero RP, H.; Alfaro, A.T.; Marques, P.T.; Brandelero, E.M. New Approach of Starch and Chitosan Films as Biodegradable Mulching. Rev. Virtual De Química 2019, 11, 686–698. [Google Scholar] [CrossRef]
- Sethi, S.; Kaith, B.S. A review on chitosan-gelatin nanocomposites: Synthesis, characterization and biomedical applications. React. Funct. Polym. 2022, 179, 105362. [Google Scholar] [CrossRef]
- Shi, C.; Tao, F.; Cui, Y. New starch ester/gelatin based films: Developed and physicochemical characterization. Int. J. Biol. Macromol. 2018, 109, 863–871. [Google Scholar] [CrossRef]
- Singh, N.; Maitra, J. Antibacterial Evaluation of Starch and Chitosan Based Polymeric Blend. J. Appl. Chem. 2015, 8, 26. [Google Scholar]
- Stanca, M.; Gaidau, C.; Zaharescu, T.; Balan, G.A.; Matei, I.; Precupas, A.; Leonties, A.R.; Ionita, G. Physico-Chemical Changes Induced by Gamma Irradiation on Some Structural Protein Extracts. Biomolecules 2023, 13, 774. [Google Scholar] [CrossRef]
- Koochakzaei, A. Determination of Sulfuric Acid Effects on Degradation and Structural Changes of Gelatin Using Fourier-Transform Infrared Spectroscopy and Peak Deconvolution Analysis. Infrared Spectrosc. 2023, 38, 5–11. [Google Scholar] [CrossRef]






| Samples | Firmness (g) | Consistency (g s−1) | Cohesiveness (|g|) | Viscosity Index (|g s−1|) |
|---|---|---|---|---|
| CA-20 | 32.1 ± 0.6 b | 279.4 ± 2.5 b | 17.2 ± 0.5 b | 158.3 ± 2.3 c |
| CA-40 | 30.5 ± 1.3 b | 262.3 ± 10.9 b | 16.4 ± 0.3 b | 137.8 ± 5.1 b |
| CA-80 | 22.9 ± 1.0 a | 215.4 ± 4.7 a | 13.2 ± 0.4 a | 90.3 ± 3.6 a |
| CA-20 | CA-40 | CA-80 |
|---|---|---|
| 76.7 ± 2.6 a | 81.3 ± 3.3 b | 85.9 ± 3.7 c |
 |  |  |
| Wavenumber (cm−1) | Amide I—Contribution (%) | ||
|---|---|---|---|
| CA-20 | CA-40 | CA-80 | |
| 1609 | 4.7 | 17.7 | 32.9 |
| 1626 | 46.5 | 45.9 | 36.5 |
| 1646 | 43.3 | 21.4 | 21.7 |
| 1664 | 5.5 | 14.9 | 8.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monroy, Y.; Rivero, S.; García, M.A. Liquid and Pressure-Sensitive Adhesives Based on Cassava Starch and Gelatin Capsule Residue: Green Alternatives for the Packaging Industry. Foods 2023, 12, 3982. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12213982
Monroy Y, Rivero S, García MA. Liquid and Pressure-Sensitive Adhesives Based on Cassava Starch and Gelatin Capsule Residue: Green Alternatives for the Packaging Industry. Foods. 2023; 12(21):3982. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12213982
Chicago/Turabian StyleMonroy, Yuliana, Sandra Rivero, and María Alejandra García. 2023. "Liquid and Pressure-Sensitive Adhesives Based on Cassava Starch and Gelatin Capsule Residue: Green Alternatives for the Packaging Industry" Foods 12, no. 21: 3982. https://0-doi-org.brum.beds.ac.uk/10.3390/foods12213982