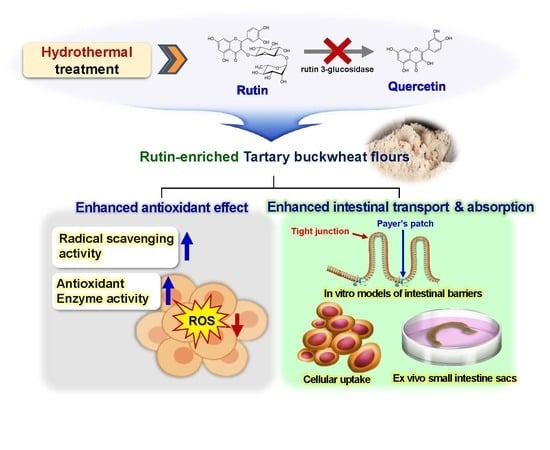
Hydrothermal Treatment Enhances Antioxidant Activity and Intestinal Absorption of Rutin in Tartary Buckwheat Flour Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extraction of Native and Hydrothermally Treated TB Flours
2.3. High-Performance Liquid Chromatography (HPLC) Analysis
2.4. Contents of Total Polyphenols and Total Flavonoids
2.5. Radical Scavenging Activity
2.6. Cell Culture and Cell Proliferation
2.7. Cellular Radical Scavenging Activity
2.8. Antioxidant Enzyme Activity
2.9. Western Blot Analysis
2.10. Cellular Uptake
2.11. Intestinal Transport
2.12. Rutin Absorption in Small Intestine Sacs
2.13. Statistical Analysis
3. Results and Discussion
3.1. Contents of Rutin, Total Polyphenols, and Total Flavonoids
3.2. Radical Scavenging Activity
3.3. Antioxidant Enzyme Activity
3.4. Cellular Uptake
3.5. In Vitro Intestinal Transport
3.6. Ex Vivo Intestinal Absorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, H.; Wei, T.; Shen, J.; Wang, M. Differences in physicochemical properties and in vitro digestibility between tartary buckwheat flour and starch modified by heat-moisture treatment. LWT-Food Sci. Technol. 2017, 86, 285–292. [Google Scholar] [CrossRef]
- Sun, X.; Li, W.; Hu, Y.; Zhou, X.; Ji, M.; Yu, D.; Fujita, K.; Tatsumi, E.; Luan, G. Comparison of pregelatinization methods on physicochemical, functional and structural properties of tartary buckwheat flour and noodle quality. J. Cereal Sci. 2018, 80, 63–71. [Google Scholar] [CrossRef]
- Fabjan, N.; Rode, J.; Košir, I.J.; Wang, Z.; Zhang, Z.; Kreft, I. Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercitrin. J. Agric. Food Chem. 2003, 51, 6452–6455. [Google Scholar] [CrossRef]
- Choi, S.Y.; Choi, J.Y.; Lee, J.M.; Lee, S.H.; Cho, E.J. Tartary buckwheat on nitric oxide-induced inflammation in RAW264.7 macrophage cells. Food Funct. 2015, 6, 2664–2670. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Piskuła, M.; Zieliński, H. Recent advances in development of gluten-free buckwheat products. Trends Food Sci. Techol. 2015, 44, 58–65. [Google Scholar]
- Zhu, F. Chemical composition and health effects of tartary buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef]
- Jiang, P.; Burczynski, F.; Campbell, C.; Pierce, G.; Austria, J.A.; Briggs, C.J. Rutin and flavonoid contents in three buckwheat species Fagopyrum esculentum, F. tataricum, and F. homotropicum and their protective effects against lipid peroxidation. Food Res. Int. 2007, 40, 356–364. [Google Scholar] [CrossRef]
- Kreft, M. Buckwheat phenolic metabolites in health and disease. Nutr. Res. Rev. 2016, 29, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, M.; Ohkawara, T.; Sato, Y.; Satoh, H.; Suzuki, T.; Ishiguro, K.; Noda, T.; Morishita, T.; Nishihira, J. Effectiveness of rutin-rich tartary buckwheat (Fagopyrum tataricum Gaertn.) ‘Manten-Kirari’ in body weight reduction related to its antioxidant properties: A randomised, double-blind, placebo-controlled study. J. Funct. Food. 2016, 26, 460–469. [Google Scholar] [CrossRef] [Green Version]
- Al-Snafi, A.E. A review on Fagopyrum esculentum: A potential medicinal plant. IOSR J. Pharm. 2017, 7, 21–32. [Google Scholar] [CrossRef]
- Lu, C.L.; Zheng, Q.; Shen, Q.; Song, C.; Zhang, Z.M. Uncovering the relationship and mechanisms of tartary buckwheat (Fagopyrum tataricum) and Type II diabetes, hypertension, and hyperlipidemia using a network pharmacology approach. PeerJ 2017, 5, e4042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Hwang, J.S.; Oh, M.S.; Lee, S.Y.; Choi, S.J. Antioxidant activity of ethanol extracts from common and tartary buckwheat milling fractions. Korean J. Food Sci. Technol. 2018, 50, 549–554. [Google Scholar]
- Kreft, I.; Fabjan, N.; Yasumoto, K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2006, 98, 508–512. [Google Scholar] [CrossRef]
- Cho, Y.J.; Lee, S.Y. Extraction of rutin from tartary buckwheat milling fractions and evaluation of its thermal stability in an instant fried noodle system. Food Chem. 2015, 176, 40–44. [Google Scholar] [CrossRef]
- Germ, M.; Árvay, J.; Vollmannová, A.; Tóth, T.; Golob, A.; Luthar, Z.; Kreft, I. The temperature threshold for the transformation of rutin to quercetin in tartary buckwheat dough. Food Chem. 2019, 283, 28–31. [Google Scholar] [CrossRef]
- Vogrinčič, M.; Timoracka, M.; Melichacova, S.; Vollmannova, A.; Kreft, I. Degradation of rutin and polyphenols during the preparation of tartary buckwheat bread. J. Agric. Food Chem. 2010, 58, 4883–4887. [Google Scholar]
- Lukšič, L.; Bonafaccia, G.; Timoracka, M.; Vollmannova, A.; Trček, J.; Nyambe, T.K.; Melini, V.; Acquistucci, R.; Germ, M.; Kreft, I. Rutin and quercetin transformation during preparation of buckwheat sourdough bread. J. Cereal Sci. 2016, 69, 71–76. [Google Scholar] [CrossRef]
- Lee, C.C.; Shen, S.R.; Lai, Y.J.; Wu, S.C. Rutin and quercetin, bioactive compounds from tartary buckwheat, prevent liver inflammatory injury. Food Funct. 2013, 4, 794–802. [Google Scholar] [CrossRef]
- Seo, S.J.; Park, C.H.; Ko, I.Y.; Jeong, Y.H.; Choi, Y.S. Comparative effects of dietary quercetin and rutin in rats fed with the Lieber-Decarli ethanol diet. Nat. Prod. 2017, 23, 222–226. [Google Scholar] [CrossRef]
- Suzuki, T.; Honda, Y.; Funatsuki, W.; Nakatsuka, K. In-gel detection and study of the role of flavonol 3-glucosidase in the bitter taste generation in tartary buckwheat. J. Sci. Food Agric. 2004, 84, 1691–1694. [Google Scholar] [CrossRef]
- Ishiguro, K.; Morishita, T.; Ashizawa, J.; Suzuki, T.; Noda, T. Antioxidative activities in rutin rich noodles and cookies made with a trace rutinosidase variety of tartary buckwheat (Fagopyrum tataricum Gaertn.), ‘Manten-Kirari’. Food Sci. Technol. Res. 2016, 22, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Fan, D.; Zhang, T. Effects of hydrothermal processing on rutin retention and physicochemical properties of tartary buckwheat enriched dough and Chinese steamed bread. Int. J. Food Sci. Tech. 2017, 52, 2180–2190. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Kim, Y.J.; Yoo, S.H.; Inglett, G.E.; Lee, S.Y. Reduction of rutin loss in buckwheat noodles and their physicochemical characterisation. Food Chem. 2012, 132, 2107–2111. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Ding, X.; Park, K.H. A process for preventing enzymatic degradation of rutin in tartary buckwheat (Fagopyrum tataricum Gaertn) flour. Food Sci. Biotechnol. 2008, 17, 118–122. [Google Scholar]
- Oh, M.S.; Oh, I.K.; Jeong, S.M.; Lee, S.Y. Optical, rheological, thermal, and microstructural elucidation of rutin enrichment in tartary buckwheat flour by hydrothermal treatments. Food Chem. 2019, 300, 125193. [Google Scholar] [CrossRef]
- Appel, H.M.; Govenor, H.L.; D’Ascenzo, M.; Siska, E.; Schultz, J.C. Limitations of folin assays of foliar phenolics in ecological studies. J. Chem. Ecol. 2001, 27, 761–778. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- De Souza, V.R.; Pereira, P.A.; da Silva, T.L.; de Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.G.; Seo, J.D.; Rhee, J.K.; Kim, C.Y. Heated apple juice supplemented with onion has greatly improved nutritional quality and browning index. Food Chem. 2016, 201, 315–319. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Peskin, A.V.; Winterbourn, C.C. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin. Chim. Acta 2000, 293, 157–166. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.Y.; Shen, S.C.; Chen, Y.C. Anti-inflammatory effect of heme oxygenase 1: Glycosylation and nitric oxide inhibition in macrophages. J. Cell. Physiol. 2005, 202, 579–590. [Google Scholar] [CrossRef]
- Zhang, X.; Song, J.; Shi, X.; Miao, S.; Li, Y.; Wen, A. Absorption and metabolism characteristics of rutin in Caco-2 cells. Sci. World J. 2013, 2013, 382350. [Google Scholar] [CrossRef]
- Go, M.R.; Yu, J.; Bae, S.H.; Kim, H.J.; Choi, S.J. Effects of interactions between ZnO nanoparticles and saccharides on biological responses. Int. J. Mol. Sci. 2018, 19, 1–16. [Google Scholar]
- Des Rieux, A.; Fievez, V.; Théate, I.; Mast, J.; Préat, V.; Schneider, Y.J. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur. J. Pharm. Sci. 2007, 30, 380–391. [Google Scholar] [CrossRef]
- Yu, J.; Kim, H.J.; Go, M.R.; Bae, S.H.; Choi, S.J. ZnO interactions with biomatrices: Effect of particle size on ZnO-protein corona. Nanomaterials 2017, 7, 377. [Google Scholar] [CrossRef] [Green Version]
- Gu, T.; Yao, C.; Zhang, K.; Li, C.; Ding, L.; Huang, Y.; Wu, M.; Wang, Y. Toxic effects of zinc oxide nanoparticles combined with vitamin C and casein phosphopeptides on gastric epithelium cells and the intestinal absorption of mice. RSC Adv. 2018, 8, 26078–26088. [Google Scholar] [CrossRef]
- Jambrec, D.; Sakač, M.; Mišan, A.; Mandić, A.; Pestorić, M. Effect of autoclaving and cooking on phenolic compounds in buckwheat-enriched whole wheat tagliatelle. J. Cereal Sci. 2015, 66, 1–9. [Google Scholar] [CrossRef]
- Lukšič, L.; Árvay, J.; Vollmannová, A.; Tóth, T.; Škrabanja, V.; Trček, J.; Germ, M.; Kreft, I. Hydrothermal treatment of tartary buckwheat grain hinders the transformation of rutin to quercetin. J. Cereal Sci. 2016, 72, 131–134. [Google Scholar] [CrossRef]
- Krkošková, B.; Mrázová, Z. Prophylactic components of buckwheat. Food Res. Int. 2005, 38, 561–568. [Google Scholar] [CrossRef]
- Christa, K.; Soral-Śmietana, M. Buckwheat grains and buckwheat products—nutritional and prophylactic value of their components—A review. Czech J. Food Sci. 2008, 26, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Chen, H.; Li, J.; Pei, Y.; Liang, Y. Antioxidant properties of tartary buckwheat extracts as affected by different thermal processing methods. LWT-Food Sci. Technol. 2010, 43, 181–185. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.-R.; Yu, J.; Choi, S.-J. Hydrothermal Treatment Enhances Antioxidant Activity and Intestinal Absorption of Rutin in Tartary Buckwheat Flour Extracts. Foods 2020, 9, 8. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9010008
Jin H-R, Yu J, Choi S-J. Hydrothermal Treatment Enhances Antioxidant Activity and Intestinal Absorption of Rutin in Tartary Buckwheat Flour Extracts. Foods. 2020; 9(1):8. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9010008
Chicago/Turabian StyleJin, Hye-Rin, Jin Yu, and Soo-Jin Choi. 2020. "Hydrothermal Treatment Enhances Antioxidant Activity and Intestinal Absorption of Rutin in Tartary Buckwheat Flour Extracts" Foods 9, no. 1: 8. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9010008