3.1. Determination of Free Amino Acids by Gas Chromatography
Chromatographic analysis provides rapid and reliable separation of chemically similar compounds in complex food matrices. Dry-cured ham includes compounds with a wide range of polarities, some of them have low polarity, like lipids, and others are strongly polar, like amino acids [
30]. GC is a high-resolution technique very useful to afford the analysis of this type of sample. Due to the low volatility, derivatization of free amino acids is performed for their analysis by GC. Several methods have been proposed in the literature. Some of them are based on multi-step procedures, which involve esterification of the carboxyl group followed by acylation of the remaining functional groups, and others are based on the formation of the silyl ethers [
31]. Several derivatizing reagents were tested, including BSTFA, N,O-bis(trimethyldilyl)acetamide (TRI-SIL/BSA), and trimethyl-chlorosilane/hexamethyldisilazane/pyridine. The most effective derivatization was achieved when using BSTFA, while TRI-SIL/BSA and trimethyl-chlorosilane/hexamethyldisilazane/pyridine showed a very low efficiency. The use of a bulkier silylating group avoided the inconvenience of multiple derivative formation observed with some amino acids [
31]. In order to improve the reaction efficiency and due to the acid hydrolysis reaction that takes place, small amounts of acetonitrile were added. Optimization of temperature and time of reaction was carried out, and the final optimal conditions were as follows: addition of 0.3 mL of acetonitrile and 0.3 mL of the derivatizing reagent (BSTFA) at 80 °C during 30 min.
Several types of columns have been used to perform the analysis of amino acids by GC, some of them being low-polarity columns of methylpolysiloxane or silicone phases, methyl 5% phenylpolisiloxane, and others medium-polarity columns with methyl 50% phenyl polysiloxane [
26,
32]. In this work, a high temperature column with methyl 50% phenyl polysiloxane stationary phase DB-17HT has been used for the determination of the amino acids.
Figure 1 shows a chromatogram of standards obtained using FID detector. The relative retention times of the amino acids to L-methionine are included in
Table 1. In general, a good separation of amino acids was obtained. It is asserted that resolution (R) of two consecutive chromatographic peaks is complete if, at full width at half height of the smallest one, they do not overlap. Thus, analyzing in more detail the chromatogram of the amino acids in
Figure 1, we can notice that complete separation was obtained for all the amino acids except for L-valine and L-β-alanine, L-glutamine and L-glutamic acid, L-histidine and L-homoarginine, with
R values of −0.163, −0.059, and −0.054, respectively.
A total of eighteen free amino acids were identified in the Iberian dry-cured ham samples.
Figure 2 shows the GC-ion trap-MS chromatogram profile in full scan mode of the trimethyl silyl ethers of the free amino acids fraction isolated according to the method proposed. The tentative assignment of the chromatographic peaks was done comparing the spectra with those from NIST 98 (National Institute of Standards and Technology, Gaithersburg, MD, USA) and WILEY 7 libraries and verified in every single case by standards.
Table 2 shows the relative retention times to methimazole, the base peak and the molecular ion for these compounds. The identified amino acids present in Iberian dry-cured ham samples were L-alanine, L-2-aminobutanoic acid, L-aspartic acid, L-cysteine, L-glutamine, L-glycine, L-histidine, L-hydroxyproline, L-isoleucine, L-leucine, L-lysine, L-methionine, L-phenylalanine, L-proline, L-serine, L-threonine, L-tyrosine, and L-valine. These eighteen amino acids were quantified using methimazole as internal standard, considering that the relative response factor to free amino acids is close to the unit.
Though there are many techniques available for the analysis of amino acids, the previous step of deproteinization is still one of the major problems. Peptides and proteins should be removed because they can interfere in the analysis and separation, as clogging the chromatographic column [
33]. Precipitation with 5-sulphosalicylic acid, followed by centrifugation, ultrafiltration, and extraction are some of the most commonly used methods of deproteinization. In meat products, separation with organic solvents like methanol, dichloromethane, and chloroform has been used [
23,
33]. In this work, a previous step of solvent extraction using n-hexane-diethyl-ether has been performed for degreasing the samples. Then, a mixture of methanol-acetonitrile was used to extract the amino acids.
For each amino acid, the calibration curves were obtained at the corresponding range of linearity. Each curve was prepared six times with a sample in which different amounts of every determined amino acid, at the levels 0, 80%, 100%, and 120%, were added. The calculated equations, area = slope × [mg kg
−1] + intercept, are presented in
Table 3. As it can be observed, a good correlation was obtained in all cases for a linear fit. The respective peak areas fitted a linear model within the indicate range shown in
Table 3. The higher the wideness of linearity range, the more reliable the linear fit is. The slopes of the calibration lines for all amino acids ranged between 314.31 and 730.34, the highest value of this was for L-threonine, and the lowest for L-methionine.
The trueness was assessed based on recovery assays that were carried out in the following way. A sample of Iberian dry-cured ham muscle was analyzed ten times by the proposed method. The obtained results for the different amino acids have been used as reference values. Then, different amounts of every determined amino acid at 80%, 100%, and 120% levels were added to the same sample, and ten replicates have been done for each case. The obtained results are shown in
Table 4. As it can be seen, recoveries lie within the range 52–164% that can be considered acceptable values according the analyzed concentration [
34] and consequently, trueness is significant.
For the determination of repeatability, replicates were done on different days and in the same laboratory.
Table 4 shows the obtained results. Relative standard deviations (RSD) range between 6.28% and 17.81%. These values of RSD are minor compared with the reference value derived from Horwitz equation. Therefore, the results for different amino acids indicate a good repeatability for the assay.
The limits of detection (LOD) and limits of quantitation (LOQ) were obtained. The LOD of the method was determined considering a signal-to-noise ratio of 3 with reference to the background noise obtained from a blank sample. LOQ was calculated considering a signal-to-noise ratio of 10. Values of LOD and LOQ are shown in
Table 4. The LOD obtained were between 7.0 and 611.74 mg kg
−1, and LOQ ranged from 27.9 to 1779.9 mg kg
−1. The lowest LOD and LOQ were for L-methionine, and the highest for L-isoleucine.
Figure 3 shows a GC–FID chromatogram of a sample of dry-cured ham. At the beginning of this amino acids chromatogram, there are some high peaks corresponding to free fatty acids, according to previously described in literature [
26]. As it is shown in
Figure 3, the free amino acids in Iberian ham muscle samples in higher amounts are L-alanine, L-glycine, L-valine, and L-proline, followed by L-leucine, L-cysteine, L-glutamine, and L-isoleucine. However, this fact disagrees with literature from other authors using a different technique [
35], in which the Iberian ham, in its ripening end step, had higher amounts of the free amino acids L-glutamic acid, L-alanine, L-leucine, and L-glycine [
35]. On the other hand, free amino acids in lesser amount were L-aspartic acid and L-methionine. Moreover, L-glutamic acid had not been detected [
35].
3.2. Evolution of Free Amino Acids during Dry-Curing Process
Concentrations of amino acids in samples of Iberian dry-cured ham at different times of the ripening process have been determined. Samples at 1, 33, 64, 99, 180, 245, 309, 363, and 435 days were taken and analyzed according to the proposed GC–FID method. The sampling days account for the three considered ripening stages, postsalting, drying, and cellar. As it has been mentioned above, eighteen free amino acids have been detected in the samples throughout the ripening period of dry-cured Iberian ham. Results are included in
Table 5. The total amount of free amino acids all along the process increases, which agrees with previous results described in the literature [
35]. From a starting value of 6387.32 mg Kg
−1 to a final one of 10320.55 mg kg
−1, the following equation can be considered: [amino acid] = 5.7767 × (days) + 4714.6;
R2 = 0.8034.
Table 6 includes the variation of percentages of free amino acids according to the ripening time. In this way, an easier form to visualize the evolution of the amino acids in the samples is achieved.
Figure 4 shows these percentages grouped for the different amino acids. As it can be seen in this figure, L-alanine is the main amino acid, with a significant decrease during postsalting and drying process, showing a maximum value of 59.56% of the total free amino acids fraction at the beginning of the process, and it stabilizes around 25.00% all along the cellar step (
Figure 4(1)). The other main amino acid found in samples is L-cysteine, which remains fixed all along the process between 23.77% and 20.31%, as shown in
Figure 4(1). L-lysine (
Figure 4(2)), L-valine, L-leucine, and L-isoleucine (
Figure 4(3)) increase all along the ripening process with a linear trend. On the other hand, the remainder components of this fraction suffer an increase that may be considered linear during postsalting and drying process to be fixed in cellar stage (
Figure 4(3–6)). Only L-aspartic acid does not show, apparently, any trend during all the process.
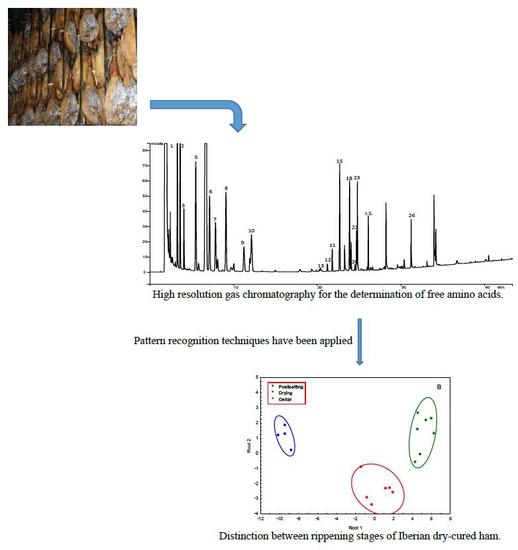
3.3. Differentiation between Ripening Stages
To differentiate between the three ripening stages considered, postsalting, drying, and cellar, some chemometric calculations have been performed using the percentages of free amino acids obtained in the analysis of the samples. By applying PCA to the data matrix, two PCs were obtained. PC1 explains 69.08% of the variance, and PC2 9.66%, accounting these two PCs for 78.64% of the total variance.
Figure 5A shows the scores plot considering the two first PCs. PC1 is strongly influenced by L-alanine, L-glycine, L-2-aminobutanoic acid, L-valine, L-leucine, L-isoleucine, L-proline, L-serine, L-aspartic acid, L-methionine, L-glutamine, L-phenylalanine, and L-lisine, and PC2 by L-cysteine, and L-histidine. The correlation between these variables is higher than 0.7. Though the samples corresponding to the different stages are not completely separated, some tendencies can be appreciated. Samples at postsalting stage are located in the scores plot at positive values of PC1, and those corresponding to cellar stage are situated at negative values of PC1. However, the samples corresponding to drying period appear spread between positive and negative values of PC1. An overlapping can be appreciated, and it could be explained considering this is a continuous process, being the first samples of drying mixed with those of postsalting and the last samples of drying mixed with the samples of cellar. This is an important consideration because in the process followed in processing plants, there are no clear criteria for transferring hams from drying to cellar stages, and the results obtained in this study can be very useful to optimize the elaboration process.
A classification rule to differentiate between the three stages was obtained by applying LDA. Using Wilks’ Lambda of 0.00532, two discriminant functions (Root 1 and 2) were obtained. Backward stepwise analysis retained the amino acids L-alanine, L-tyrosine, L-glutamine, L-proline, L-2-aminobutanoic acid, L-cysteine, and L-valine that can be considered the most discriminant variables. The classification functions are shown in
Table 7. A complete separation between the three stages was obtained, denoting that the considered variables are powerful descriptors to differentiate between the samples from three dry-curing periods considered, postsalting, drying, and cellar.