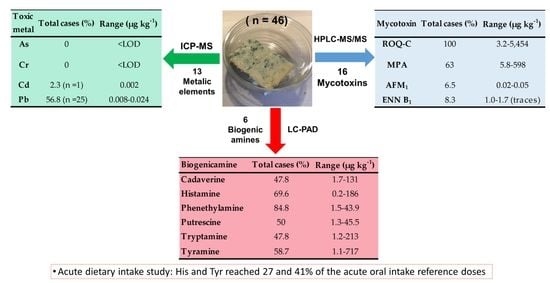
3.1. Method Validation
All of the methods provided sufficient linearity, with the determination coefficients (R2) higher than 0.99 for all of the tested analytes.
The mycotoxin method showed satisfactory recoveries (R = 91–117%) and intra-day repeatability (RSDs < 20%, with the exception of 29% for BEA) and acceptable measurement uncertainty for most of the target analytes (6–20%), whereas, higher uncertainty values (>20%) were determined for OTA, CVD, ENNs, and BEA (
Table S1). These data were obtained from the validation using brine-matured fresh cheese as the blank matrix. Compared to other reported methods, the sensitivity of the developed method was satisfactory—the LODs for AFM
1, MPA, and ROQ C (0.004, 0.12, and 0.47 µg kg
−1) were at least 5 times lower compared to other methods, for example, as described by [
7], whereas the detection limits for these mycotoxins were 0.02, 3.00, and 4.00 µg kg
−1.
The brine-matured fresh salad cheese was successfully used as the blank matrix also for quality control of HPLC-PAD method, as this type of cheese has been reported to contain notably lower BA levels compared to other cheese varieties [
10,
27]. Satisfactory recovery (83–117%), precision, and uncertainty (RSD < 16%) of the HPLC-PAD method were determined from the performance assessment studies involving the analysis of samples fortified with a standard additive at three concentration levels, five replicates at each level, with the analyses performed over two days (
Table S2). The method uncertainties were determined from the control chart data and were in the range of 26–32% (
Table S2).
The selectivity of the HPLC-PAD method was tested by evaluating the effects of analyte signals and interfering signals generated by the matrix. As shown in
Figure 1, no peaks interfered with the analyte signals. Although the retention times of Cad and His were close, selectivity was sufficient, as determined at the highest calibration point (75 mg kg
−1).
The stability of BAs was evaluated within a single sequence by measuring the change of analyte concentrations over one sequence (~20 injections). Samples were stored in the autosampler at 15 °C in the dark for the analysis (
Figure S1 of the Supplementary Material S1). While the concentrations of Phe, Try, and Tyr showed slight decrease during the sequence and the concentrations of other BAs increased by a small amount, the fluctuations were only in the range of 0.2–2.6%, indicating that the analytes remained stable during the sequence, thus allowing their proper determination by the developed method. In addition, the content of BAs in some cases exceeded the maximal value (75 mg kg
−1) of the linearity range. Therefore, if the concentration exceeded the calibration range, the samples were diluted and the actual levels were recalculated accordingly. This procedure also allowed to avoid specific non-linear calibration issues that may occur at the high concentrations used in BA testing involving derivatisation with dansyl chloride [
28].
The performance parameters of ICP-MS method for heavy metals analysis are summarised in
Table S3, which indicate sufficient recovery (R < 120%), repeatability (RSDs < 20%) according to the requirements of EU legislation [
29]. While the sensitivity was lower than achieved by [
18], the LOD values were sufficient, compared to the EU regulations for the determination of toxic elements.
3.2. The Occurrence of Mycotoxins in the Tested Blue Cheeses
The developed HPLC-MS/MS method enabled simultaneous analysis of mycotoxins produced by starter cultures (ROQ C, MPA) and specific strains that can indicate spoilage within one sample injection. As expected, all of the samples contained detectable concentrations of ROQ C ranging between 3.2 and 5.454 µg kg
−1 (
Table 3).
The lowest ROQ C content was determined in Morbier AOC French cheese variety, which was analysed for comparison, reflecting the fact that this semi-hard cheese does not belong to the blue-veined variety. As noted in
Table 3, the concentrations of ROQ C in cheeses from Poland ranged from very low to medium high levels. The lowest and highest concentrations (5.5 and 790 µg kg
−1) were associated with specific cheese varieties, namely, brie (white-mould cheese) and blue-veined cheese. The mean ROQ C levels determined in other blue cheese samples from Poland ranged between 250 and 590 µg kg
−1. The highest content of ROQ C was found in Blue Stilton varieties, with the mean concentration of 3675 µg kg
−1.
Latvian Bio-Blue, three Italian, and one German cheese, as well as the majority of Danish blue cheeses also contained rather high contents of ROQ C. The differences of ROQ C content could be attributed to different concentrations of fungal starter cultures added to cheeses during their production, rather than differences in ripening [
7]. An earlier study, indicated 1.5–12 times higher concentrations of ROQ C (0.8 to 12 mg kg
−1) found in blue cheeses from the Finnish market as compared to that of the current study [
6]. However, similarly to our test results, French cheeses in that study showed a widest variation, whereas only one of the total of twenty-one samples from the Finnish market was positive for MPA, with the concentration of 300 µg kg
−1 [
7].
The current study of forty-six cheese varieties marketed in Latvia showed co-occurrence of MPA with ROQ C in 63% of the samples (
n = 29), with the concentrations of MPA ranging between 5.8 and 599 µg kg
−1 (
Table 3). The highest concentrations of MPA (>190 µg kg
−1) were found in the Danish blue (
n =3) and Roquefort type (
n = 3) cheeses, whereas eighteen of the samples, mainly originating from Poland, Italy, Germany, Latvia, and in three cases from Denmark contained relatively low amounts of MPA (<50 µg kg
−1). The concentrations of MPA in two of the Danish cheese samples analysed during our study were in agreement with those reported by other authors, reaching 500 µg kg
−1 of MPA in these cheese varieties according to HPLC-UV/Vis analysis [
30]. Our results were comparable with the mean mycotoxin levels reported for blue cheese varieties by [
7], who tested more than eighty commercial cheese samples and evaluated the evolution of ROQ C and MPA concentrations in the samples during different ripening stages. The determined mean average levels of ROQ C and MPA from those studies were in the range of 11–14,125 µg kg
−1 and 15–6190 µg kg
−1, respectively. In that study, the cheese samples were categorised according to the origin of milk. While higher levels of MPA were present in goat milk and the lowest levels were observed in cheeses produced from blended milk (there was little change for ROQ C), no conclusions about correlation could be made due to the scarcity of data about different cheese categories. That was also noted during our study, as the cow milk samples showed rather wide distribution of mycotoxin levels compared to the mixed or other milk types. Furthermore, according to the study by [
7], MPA concentrations have a tendency to increase with ripening time.
Most of the recently reported methods have been focussed on a particular mycotoxin or group of mycotoxins such as OTA [
8,
31], penitrems A to F [
9], whereas AFM
1 was mainly studied together with ROQ C and MPA [
6,
7,
32]. The transformation of labile mycotoxins (PA, PR toxin) into metabolites during storage has also been characterised, whereas an absence of those mycotoxins in manufactured blue cheeses was reported [
33]. Cacmacki et al. (2015) reported rather high concentrations of PA (0.2–43.6 mg kg
−1), as well as similar levels of ROQ C (0.4–47.0 mg kg
−1) and MPA (0.1–23.1 mg kg
−1) in Turkish traditional cheese, which was ripened for a three-month period in the presence of
P. roqueforti fungal cultures [
34].
The current study did not indicate elevated concentrations of PA or other mycotoxins that are especially associated with spoilage (e.g., SBL, OTs, CVD, and others). AFM
1 was also below the detection level in most of the tested samples, indicating a good quality of milk used for the cheese production, except for three out of five Italian Gorgonzola samples that contained detectable concentrations of AFM
1, from 0.02 µg kg
−1 up to the maximum permitted level of this toxin in milk (0.05 µg kg
−1). For comparison, the study [
7] and other earlier reports did not report detectable levels of AFM
1.
An interesting finding within the current study was the detection of one emerging
Fusarium mycotoxin (ENN B), which was present at low concentrations (1.0–1.7 µg kg
−1) in four different cheese samples made of cow milk—two French cheeses, one sample from Poland, and processed blue veined cheese from Lithuania. No other ENNs or BEA were found. This is the first study indicating traces of this emerging mycotoxin in blue mould cheese made of cow milk. A recent paper by Polish researchers based on HPLC-MS/MS method revealed low levels of ENN B (0.0055–0.0121 µg kg
−1) in eighteen out of the total of twenty tested samples of sheep milk [
35]. Taking into account the recent report by Tolosa et al. (2019) of ENN B as the most common emerging mycotoxin in animal feed [
36], it is possibly worth to continue studies of emerging
Fusarium mycotoxins in food and feed, including foods of animal origin, such as dairy products.
3.3. The Occurrence of Biogenic Amines in Blue Cheeses
The results of BA determination are summarised in
Table 4 and
Figure 2 as the frequency of BA occurrence in the tested cheeses at different concentrations.
Concentrations below LODs were found in only one out of six tested Gorgonzola samples. Two samples (Italian Montagnolo and Lithuanian processed blue cheese) contained His at concentrations above the method LOQ (0.2 and 0.3 mg kg
−1). However, these two samples also contained Tyr (440 and 50.3 mg kg
−1). The other tested cheese samples were positive for one to six BAs, with the individual concentrations ranging from just above the reporting level (>1 mg kg
−1) up to 719 mg kg
−1, while the total content of BAs ranged between 5.5 and 824 mg kg
−1. Almost quarter (
n = 12) of the tested cheese samples contained under 10 mg kg
−1 of BAs (
Figure 2).
In other samples, one or two BAs exceeded that level. Five samples, including two Cambozola, Monatanaglo, Gorgonzola and Ranka Blue cheeses, and Latvian blue cheese were found with Tyr, Try, and His concentrations exceeding 150 mg kg
−1 (
Table 4). The rather wide differences of BA distribution among the tested cheese samples were influenced by the different geographical origins and types of processing (
Figure 2). A risk evaluation of BAs in different food products was reported by EFSA in 2011, covering more than 600 blue cheese samples from different EU countries [
27]. This EFSA report indicated that cadaverine (Cad), as BA of low oral toxicity, was the most prevailing contaminant of this type in 23% of the reported samples (mean levels: 83.1–121 mg kg
−1). Within this study, the presence of His was detected in 15% of the samples at the concentration range of 21.8–63.8 mg kg
−1, Tyr was detected in 17% of the cases (63.2–104 mg kg
−1), and Put was also found in 17% of the cases (20.9–62.2 mg kg
−1). Other, less toxic BAs (e.g., Try, Phe) have been detected at rather low concentrations, but occurred frequently [
13,
27]. According to deterministic risk characterisation based on the hazard level (NOAEL) of 50 mg set for His by EFSA and the 270 g per day upper limit of consumption, 185 mg kg
−1 is the highest tolerated concentration of His in healthy adults not using antihistamine drugs [
27]. Benkeroum (2016) discussed different approaches to risk evaluation, used in Australia and other countries, which all included levels ranging from 100 to 400 mg kg
−1 as the maximum allowed levels according to EFSA recommendations for healthy adults not using antihistamine drugs [
10]. However, these values should be re-evaluated, taking into account the different consumption data in Europe, and this task is part of the dietary assessment in the current study.
Phe was the most prevalent BA, detected in more than 84% of the tested forty-six cheese samples in the current study. Similarly, to other BAs, Phe is known to act as a neurotransmitter, which can initiate hypertension and headache, with a threshold toxic level of 30 mg in healthy adults not under medications [
10]. The concentration of Phe ranged between 1.5 and 21.6 mg kg
−1 in the tested blue mould cheeses, with an exception of 43.9 mg kg
−1 determined in processed blue cheese from Lithuania. For comparison, according to the recent review [
10], the concentrations of Phe ranged from below the limit of detection to 39.7 mg kg
−1 found by a research study in 2003, whereas, more recent studies of cheese from sheep’s milk found Phe levels up to 69 mg kg
−1 [
14]. An earlier report by EFSA claimed the detection of Phe in 54% of the cases, which is less frequently than in the current study, while the concentrations for Phe in the current study were quite similar to those reported by an EFSA survey of blue veined cheese with the mean and the highest concentrations reaching 5.5 and 39.5 mg kg
−1, respectively [
27].
In the current study, His was the second most prevalent BA, which was found at concentrations between 0.2 and 186 mg kg
−1 in 69.6% of the tested cheese samples. Mayer et al. (2018) recently reported the detection of His in 19 out of 31 samples (61% of samples) at the mean concentration of 36.6 mg kg
−1, with the maximum level of 255.3 mg kg
−1 [
13]. This study determined that higher levels of His may be presented in acid-curd and hard cheeses, with the maximum concentration of 1159.7 mg kg
−1.
Madejska et al. (2018) reported potential safety issues during the storage of white mould and blue-veined cheeses purchased in Poland [
37]. In the case of white mould cheese, an increase of His content from 150 to 400 mg kg
−1 was found after long term storage in a conventional refrigerator at +4 °C. The level of His in Gorgonzola Piccante reached 730 ± 20 mg kg
−1 after storage for forty-two days at +22 °C. The authors of that study noted rather high levels of His (162.63 ± 23.57 mg kg
−1) in raw Gorgonzola Piccante control samples, implying improper storage conditions during the processing or ripening of the tested cheese sample. These observations by Polish researchers were in agreement with the current research, where six different Gorgonzola samples were included. Four of the Gorgonzola varieties examined during our study contained His in the range from <LOD to 63.6 mg kg
−1, but two samples of Gorgonzola Piccante showed elevated His contents: 140 and 186 mg kg
−1.
Tyramine, an aromatic amine arising from the decarboxylation of tyrosine is commonly present in cheese samples at higher concentrations compared to other BAs. The typical concentrations in the range from 100 to 800 mg kg
−1 [
10] are acceptable for healthy adults. In our study, 58.7% of the samples (
n = 27) were positive for Tyr at concentrations generally ranging from 1.1 to 110 mg kg
−1 in 25 of the samples, whereas, two samples of Italian cheeses showed rather high concentrations of this BA: the previously reported Montagnolo cheese with Tyr content of >440 mg kg
−1 and Cambozola with garlic containing >700 mg kg
−1 of Tyr. These data are in agreement with the results reported by other researchers, which also noted high Tyr levels in Italian and other mould-ripened cheeses: 627 mg kg
−1 [
38], 308.7 mg kg
−1 and 308.65–585.47 mg kg
−1 in cheese made from sheep milk [
14].
The levels of Cad, Put, and Try in the tested cheese samples were very similar, with 22 to 23% of samples containing detectable concentrations of these BAs, ranging between 1.2 and 213 mg kg
−1. Compared to His and Tyr, these three BAs have low acute toxicity. The acceptable levels of Cad and Put in cheese are 540 mg kg
−1 and 180 mg kg
−1, respectively [
10]. Our data indicated considerably lower levels of Put and Cad, with the maximum content of these BAs equal to 45.5 and 131 mg kg
−1 in Italian and Polish cheese samples. A Gorgonzola cheese from Italy had a rather high (197 mg kg
−1) content of Try in our study as well. For comparison, Mayer et al. (2018) found up to 110 mg kg
−1 of Try only in 1 out of 31 tested commercial cheese samples, whereas Put and Cad where found in 29 and 16 out of the total of 31 blue cheeses, with the maximum levels of 527 and 830 mg kg
−1, respectively [
13].
3.4. The Occurrence of Chemical Elements, Microelements, and Toxic Metals
Only forty-four of the cheese samples were selected for heavy metal analysis, as two cheese samples from Poland were insufficient for these tests. The results are shown in
Table 5. Among the determined heavy metals, cobalt (Co), nickel (Ni), copper (Cu), and arsenic (As) were below the levels of detection (
Table 5). Christophoridis et al. (2019) also reported the absence of as even at trace levels (<1.1 µg kg
−1 wet weight (ww)) in blue mould cheese marketed in Thessaloniki, Greece [
18]. The absence of As in this case was reasonably expected, as its most commonl source is water from polluted geographic regions that is consumed by animals [
39]. In fact, the study by Christophoridis et al. (2019) is the only study of heavy metals in blue mould cheeses. That study reported mean concentrations of Cu and Ni reaching 290 and 485 µg kg
−1 ww in blue mould cheese, whereas Ni levels were associated with contamination through storage tankers and milking equipment within the primary processing stages.
Similarly, to lead (Pb), cadmium (Cd) is a suspected carcinogen, which may be present in air from industrial emissions and contaminate water. In the current study of cheese samples from the Latvian market, Cd was detected in only one sample of Roquefort cheese at the LOD level (0.002 mg kg
−1 ww). Starska et al. (2011) also reported similar levels of Cd in dairy products, including cheeses from the Poland market [
40]. Christophoridis et al. (2019) did not find traces of Cd or other toxic metals in blue cheese, whereas the concentrations in Feta cheese and Mozzarella marketed in Thessaloniki were comparable to that found in our study (1.15 and 2.95 µg kg
−1 ww). Christophoridis et al. (2019) have provided a concise summary of recently published methods and test results of heavy metals in cheese samples. Thus, a wide range of Cd levels from 0.3 to 600 µg kg
−1 ww have been reported in different cheese samples from Greece, Italy, Iran, and Egypt.
In the current study, lead (Pb) was found in more than 50% of the tested cheese samples (
n = 25), with the concentrations ranging between 0.007 and 0.024 mg kg
−1 ww. Most of the samples contained Pb at trace levels between the LOD and 0.017 mg kg
−1, while only three samples (one DorBlu, Latvian blue cheese, and one Blue Stilton variety) contained concentrations around the maximum permissible level (0.02 mg kg
−1 or 20 µg kg
−1) established for milk according to the EU Regulation 1881/2006 [
3]. Cristophoridis et al. (2019) found a considerably wider range of Pb levels (0.014–10.7 mg kg
−1 ww) in cheese samples from Egypt, Brazil, Turkey, and other regions.
Tin (Sn) is another toxic metal, which may migrate from metal surfaces in machinery into milk, resulting in a rather high content (39.5 mg kg
−1 ww) determined in Roquefort cheese, which was sold under a supermarket brand, whereas the other positive samples (
n = 12) contained only traces of this element around the detection level. From the detected micronutrients, Zn was determined at levels up to 39.5 mg kg
−1 ww, similar to those reported by other authors concerned with the bioavailability of micronutrients in the soil used for dairy farming and migration from machinery into milk [
19]. The other micronutrients (Mn, Fe) were present in the samples at very low concentrations, which were comparable to those reported by [
19].