The Effects of Oxidation on the Antithrombotic Properties of Tea Lipids against PAF, Thrombin, Collagen, and ADP
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
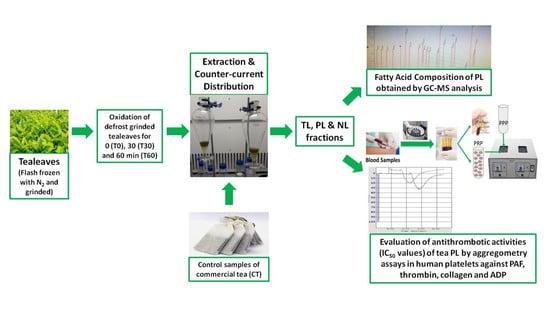
2.2. Samples of Tea Assessed before and after Oxidation Processing
2.3. Extraction and Isolation of Total, Neutral, and Polar Lipids From Tea Leaves before (0 min) and after 30 and 60 min of Oxidation
2.4. Human Platelet Aggregation Studies against PAF, Thrombin, Collagen, and ADP of Lipid Extracts from Tea Leaves before (0 min) and after 30 and 60 min of Oxidation
2.5. Gas Chromatography-Mass Spectrometry of Polar Lipids from Tea Leaves before (0 min) and after 30 and 60 min of Oxidation
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heaney, S.; Koidis, A.; Morin, J.M. Tea and flavoured tea. In Handbook of Food Authenticity: A Guide to Food Authenticity Issues and Analytical Solutions; Morin, J.F., Lees, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 315–331. ISBN 978-2-9566303-0-2. [Google Scholar]
- Sumpio, B.E.; Cordova, A.C.; Berke-Schlessel, D.W.; Qin, F.; Chen, Q.H. Green tea, the “Asian paradox,” and cardiovascular disease. J. Am. Coll. Surg. 2006, 202, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.V.; Liu, D. Green tea catechins and cardiovascular health: An update. Curr. Med. Chem. 2008, 15, 1840–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anan, T. The Lipids of Tea. JARQ 1983, 16, 253–257. [Google Scholar]
- Roberts, G.R. Polar lipid composition of the leaves and seeds from the tea plant (Camellia sinensis L). J. Sci. Food Agric. 1974, 25, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Burgos, A.; Ma, L.; Zhang, Q.; Tang, D.; Ruan, J. Lipidomics analysis unravels the effect of nitrogen fertilization on lipid metabolism in tea plant (Camellia sinensis L.). BMC Plant Biol. 2017, 17, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhuyan, L.P.; Mahanta, P.K. Studies on fatty acid composition in tea Camellia sinensis. J. Sci. Food Agric. 1989, 46, 325–330. [Google Scholar] [CrossRef]
- Shen, S.R.; Yu, H.N.; Chen, P.; Yin, J.J.; Xiong, Y.K. Fatty acids in tea shoots (Camellia sinensis (L.) O. Kuntze) and their effects on the growth of retinal RF/6A endothelial cell lines. Mol. Nutr. Food Res. 2007, 51, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Croft, K.D. Tea flavonoids and cardiovascular health. Mol. Asp. Med. 2010, 31, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Sagesaka-Mitane, Y.; Miwa, M.; Okada, S. Platelet aggregation inhibitors in hot water extract of green tea. Chem. Pharm. Bull. (Tokyo) 1990, 38, 790–793. [Google Scholar] [CrossRef] [Green Version]
- Sugatani, J.; Fukazawa, N.; Ujihara, K.; Yoshinari, K.; Abe, I.; Noguchi, H.; Miwa, M. Tea polyphenols inhibit acetyl-CoA:1-alkyl-sn-glycero-3-phosphocholine acetyltransferase (a key enzyme in platelet-activating factor biosynthesis) and platelet-activating factor-induced platelet aggregation. Int. Arch. Allergy Immunol. 2004, 134, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Janssen, K.; Mensink, R.P.; Cox, F.J.; Harryvan, J.L.; Hovenier, R.; Hollman, P.C.; Katan, M.B. Effects of the flavonoids quercetin and apigenin on hemostasis in healthy volunteers: Results from an in vitro and a dietary supplement study. Am. J. Clin. Nutr. 1998, 67, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Lee, V.S.; Tseng, Y.L.; Chang, K.C.; Chen, K.B.; Chen, Y.L.; Li, C.Y. Gallic acid attenuates platelet activation and platelet-leukocyte aggregation: Involving pathways of Akt and GSK3β. Evid. Based Complement. Altern. Med. 2012, 2012, 683872. [Google Scholar] [CrossRef] [Green Version]
- Cyboran, S.; Strugała, P.; Włoch, A.; Oszmiański, J.; Kleszczyńska, H. Concentrated green tea supplement: Biological activity and molecular mechanisms. Life Sci. 2015, 126, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.B.; Iatrou, C.; Frangia, C.; Demopoulos, C.A. The implication of platelet activating factor in cancer growth and metastasis: Potent beneficial role of PAF-inhibitors and antioxidants. Infect. Disord. Drug Targets 2009, 9, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Holy, E.W.; Forestier, M.; Richter, E.K.; Akhmedov, A.; Leiber, F.; Camici, G.C.; Mocharla, P.; Lüscher, T.F.; Beer, J.H.; Tanner, F.C. Dietary α-linolenic acid inhibits arterial thrombus formation, tissue factor expression, and platelet activation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1772–1780. [Google Scholar] [CrossRef] [Green Version]
- Freese, R.; Mutanen, M. Alpha-linolenic acid and marine long-chain n-3 fatty acids differ only slightly in their effects on hemostatic factors in healthy subjects. Am. J. Clin. Nutr. 1997, 66, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Nunez, D.; Randon, J.; Gandhi, C.; Siafaka-Kapadai, A.; Olson, M.S.; Hanahan, D.J. The inhibition of platelet-activating factor-induced platelet activation by oleic acid is associated with a decrease in polyphosphoinositide metabolism. J Biol. Chem. 1990, 265, 18330–18338. [Google Scholar]
- Bazán-Salinas, I.L.; Matías-Pérez, D.; Pérez-Campos, E.; Pérez-Campos Mayoral, L.; García-Montalvo, I.A. Reduction of platelet aggregation from ingestion of oleic and linoleic acids found in Vitis vinifera and Arachis hypogaea Oils. Am. J. Ther. 2016, 23, e1315–e1319. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [Green Version]
- Nasopoulou, C.; Smith, T.; Detopoulou, M.; Tsikrika, C.; Papaharisis, L.; Barkas, D.; Zabetakis, I. Structural elucidation of olive pomace fed sea bass (Dicentrarchus labrax) polar lipids with cardioprotective activities. Food Chem. 2014, 145, 1097–1105. [Google Scholar] [CrossRef]
- Nasopoulou, C.; Tsoupras, A.B.; Karantonis, H.C.; Demopoulos, C.A.; Zabetakis, I. Fish polar lipids retard atherosclerosis in rabbits by down-regulating PAF biosynthesis and up-regulating paf catabolism. Lipids Health Dis. 2011, 10, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoupras, A.; Lordan, R.; Shiels, K.; Saha, S.K.; Nasopoulou, C.; Zabetakis, I. In vitro antithrombotic properties of Salmon (Salmo salar) phospholipids in a novel food-grade extract. Mar. Drugs 2019, 17, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoupras, A.; Lordan, R.; Demuru, M.; Shiels, K.; Saha, S.K.; Nasopoulou, C.; Zabetakis, I. Structural elucidation of irish organic farmed Salmon (Salmo salar) polar lipids with antithrombotic activities. Mar. Drugs 2018, 16, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoupras, A.; O’Keeffe, E.; Lordan, R.; Redfern, S.; Zabetakis, I. Bioprospecting for antithrombotic polar lipids from salmon, herring, and boarfish by-products. Foods 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lordan, R.; O’Keeffe, E.; Tsoupras, A.; Zabetakis, I. Total, neutral, and polar lipids of brewing ingredients, by-products and beer: Evaluation of antithrombotic activities. Foods 2019, 8, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lordan, R.; O’Keeffe, E.; Heffernan, H.; Dowling, D.; Mullally, M.; Tsoupras, A.; Zabetakis, I. In vitro antithrombotic properties of ale, lager, and stout. Food Biosci. 2019, 28, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Koukouraki, P.; Tsoupras, A.; Sotiroudis, G.; Demopoulos, C.A.; Sotiroudis, T.G. Antithrombotic properties of Spirulina extracts against platelet-activating factor and thrombin. Food Biosci. 2020. in print (accepted for publication). [Google Scholar]
- Tsoupras, A.B.; Demopoulos, C.A.; Pappas, K.M. Platelet-activating factor detection, metabolism and inhibitors in the ethanologenic bacterium Zymomonas mobilis. Eur. J. Lipid Sci. Technol. 2012, 114, 123–133. [Google Scholar] [CrossRef]
- Ercisli, S.; Orhan, E.; Ozdemir, O.; Sengul, M.; Gungor, N. Seasonal variation of total phenolic, antioxidant activity, plant nutritional elements, and fatty acids in tea leaves (Camellia sinensis var. sinensis clone Derepazari 7) Grown in Turkey. Pharm. Biol. 2008, 46, 683–687. [Google Scholar] [CrossRef]
- Wright, A.J.; Fishwick, M.J. Lipid degradation during manufacture of black tea. Phytochemistry 1979, 18, 1511–1513. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galanos, D.S.; Kapoulas, V.M. Isolation of polar lipids from triglyceride mixtures. J. Lipid Res. 1962, 3, 134–136. [Google Scholar]
- Tsoupras, A.; Zabetakis, I.; Lordan, R. Platelet aggregometry assay for evaluating the effects of platelet agonists and antiplatelet compounds on platelet function in vitro. MethodsX 2018, 6, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, T.; Karantonis, H.C.; Fragopoulou, E.; Demopoulos, C.A. One-step separation system for the main phospholipids, glycolipids, and phenolics by normal phase HPLC. Application to polar lipid extracts from olive and sunflower oils. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 137–149. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Antonopoulou, S.; Tsoupras, A.; Tsantila, N.; Grypioti, A.; Gribilas, G.; Gritzapi, H.; Konsta, E.; Skandalou, E.; Papadopoulou, A.; et al. Antiatherogenic properties of red/white wine, musts, grape-skins, and yeast. Chem. Phys. Lipids 2004, 130, 66. [Google Scholar]
- Cui, L.; Decker, E.A. Phospholipids in foods: Prooxidants or antioxidants? J. Sci. Food Agric. 2016, 96, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.A.; Fuller, E.; Yang, W. Identification of native catechin fatty acid esters in green tea (Camellia sinensis). J. Agric. Food Chem. 2013, 61, 11484–11493. [Google Scholar] [CrossRef]
- Okal, A.; Okinda Owuor, P.; Kamau, D.; Manguro, L. Variations of fatty acids levels in young shoots of clonal tea with location of production and nitrogenous fertilizer rates in the Kenya highlands. JAST 2012, 14, 1543–1554. [Google Scholar]
- Maciejewska, D.; Lukomska, A.; Jakubczyk, K.; Baranowska-Bosiacka, I.; Stachowska, E.; Chlubek, D.; Gutowska, I. The content of linoleic and alpha-linolenic acid in different types of Yerba Mate, depending on country of origin and the conditions of the infusion. Pomeranian J. Life Sci. 2015, 61, 90–93. [Google Scholar] [CrossRef]
- Guil, J.L.; Torija, M.E.; Giménez, J.J.; Rodríguez, I. Identification of fatty acids in edible wild plants by gas chromatography. J. Chromatogr. A 1996, 719, 229–235. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Karaosmanoglu, H.; Kilmartin, P.A. Tea extracts as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2015; pp. 219–233. [Google Scholar]
- Hudson, B.J.F.; Mahgoub, S.E.O. Synergism between phospholipids and naturally occurring antioxidants in leaf lipids. J. Sci. Food Agric. 1981, 32, 208–210. [Google Scholar] [CrossRef]
- Sirk, T.W.; Friedman, M.; Brown, E.F. Molecular binding of black tea theaflavins to biological membranes: Relationship to bioactivities. J. Agric. Food Chem. 2011, 59, 3780–3787. [Google Scholar] [CrossRef]
- Mouhid, L.; Corzo-Martínez, M.; Torres, C.; Vázquez, L.; Reglero, G.; Fornari, T.; Ramírez de Molina, A. Improving in vivo efficacy of bioactive molecules: An overview of potentially antitumor phytochemicals and currently available lipid-based delivery systems. J. Oncol. 2017, 2017, 7351976. [Google Scholar] [CrossRef] [Green Version]
- Kidd, P.N. Bioavailability and activity of phytosome complexes from botanical polyphenols: The silymarin, curcumin, green tea, and grape seed extracts. Altern. Med. Rev. 2009, 14, 226–246. [Google Scholar]
- Yang, L.; Cao, Y.; Chen, J.N.; Chen, Z.Y. Oxidative stability of conjugated linolenic acids. J. Agric. Food Chem. 2009, 57, 4212–4217. [Google Scholar] [CrossRef]
- Chung, T.Y.; Kuo, P.C.; Liao, Z.H.; Shih, Y.E.; Yang, M.L.; Cheng, M.L.; Wu, C.C.; Tzen, J.T.C. Analysis of lipophilic compounds of tea coated on the surface of clay teapots. J. Food Drug Anal. 2015, 23, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Ravichandran, R.; Parthiban, R. Lipid occurrence, distribution and degradation to flavour volatiles during tea processing. Food Chem. 2000, 68, 7–13. [Google Scholar] [CrossRef]


| Tea Sample | TL * | NL * | PL * |
|---|---|---|---|
| CT | 8.1 ± 1.5 | 0.8 ± 0.3 | 7.3 ± 1.2 |
| T(0) | 9.6 ± 4.9 | 1.6 ± 0.7 | 8.0 ± 5.6 |
| T(30) | 9.6 ± 0.6 | 0.3 ± 0.1 | 9.3 ± 0.3 |
| T(60) | 10.7 ± 4.0 | 0.9 ± 2.0 | 9.8 ± 2.0 |
| Fatty Acid | 0M | 30M | 60M | CT |
|---|---|---|---|---|
| 14:0 | 0.700 ± 0.138 a | 0.135 ± 0.102 ab | 0.122 ± 0.003 b | 0.227 ± 0.019 ab |
| 14:1 | ND | ND | ND | 0.064 ± 0.013 |
| 15:0 | 0.120 ± 0.012 a | 0.047 ± 0.002 b | ND | 0.091 ± 0.003 ab |
| 16:0 | 24.60 ± 0.355 ab | 19.41 ± 1.117 a | 23.38 ± 0.947 ab | 28.11 ± 2.014 b |
| 16:1 c9 | 1.927 ± 0.033 ab | 1.461 ± 0.034 a | 1.752 ± 0.073 ab | 2.217 ± 0.080 b |
| 17:0 | 0.352 ± 0.010 b | 0.274 ± 0.017 ab | 0.020 ± 0.025 a | 0.332 ± 0.022 ab |
| 18:0 | 6.408 ± 0.241 ab | 8.496 ± 0.173 b | 7.930 ± 0.124 ab | 6.013 ± 0.099 a |
| 18:1 c9 | 9.965 ± 0.149 ab | 11.95 ± 0.274 b | 8.039 ± 0.114 ab | 7.840 ± 0.200 a |
| 18:1 c11 | 1.500 ± 0.115 b | 1.116 ± 0.048 ab | 0.787 ± 0.095 a | 1.184 ± 0.043 ab |
| 18:2 c9, c12 | 19.48 ± 0.222 a | 22.34 ± 0.262 a | 22.37 ± 0.247 a | 20.38 ± 0.839 a |
| 18:3 c9, c12, c15 | 29.78 ± 0.011 ab | 30.84 ± 0.529 ab | 34.22 ± 0.282 b | 27.44 ± 0.942 a |
| 20:0 | 0.343 ± 0.064 a | 0.442 ± 0.038 a | ND | 0.209 ± 0.032 a |
| 20:1 c9 | 1.105 ± 0.044 b | 0.698 ± 0.030 ab | ND | 0.379 ± 0.051 a |
| 20:4 c5, c8, c11, c14 | 0.344 ± 0.0109 ab | 0.397 ± 0.018 b | ND | 0.244 ± 0.033 a |
| 20:5 c5, c8, c11, c14, c17 | 0.732 ± 0.016 b | 0.655 ± 0.029 ab | 0.574 ± 0.050 ab | 0.339 ± 0.073 a |
| 22:0 | 1.052 ± 0.015 b | ND | ND | 0.319 ± 0.044 a |
| 22:5 c7, c10, c13, c16, c19 | ND | ND | ND | 0.375 ± 0.050 |
| 22:6 c4, c7, c10, c13, c16, c19 | 1.390 ± 0.113 b | 1.370 ± 0.155 ab | 0.518 ± 0.142 a | 1.122 ± 0.106 bc |
| ω3 | 31.90 ± 0.215 ab | 32.86 ± 0.433 ab | 35.56 ± 0.176 b | 29.28 ± 1.060 a |
| ω6 | 19.83 ± 0.228 a | 22.74 ± 0.271 b | 22.37 ± 0.247 ab | 20.62 ± 0.860 ab |
| ω6/ω3 | 0.621 ± 0.011 | 0.692 ± 0.017 | 0.629 ± 0.010 | 0.704 ± 0.055 |
| SFA | 32.40 ± 0.532 ab | 28.80 ± 0.936 a | 31.64 ± 0.829 ab | 35.28 ± 1.905 b |
| MUFA | 15.55 ± 0.141 b | 15.28 ± 0.299 ab | 10.58 ± 0.051 a | 11.90 ± 0.287 ab |
| PUFA | 51.73 ± 0.413 ab | 55.60 ± 0.681 ab | 57.68 ± 0.805 b | 49.90 ± 1.873 a |
| Inhibitory Effect * | Agonistic Effect ** | ||||
|---|---|---|---|---|---|
| Standard | PAF | Thrombin | Collagen | ADP | - |
| Aspirin | 13.1 ± 5.0 (291.4 ± 111.8) # | 3.5 ± 1.8 (77.4 ± 39.3) | 3.0 ± 2.0 (66.4 ± 52.1) | 3.6 ± 0.8 (79.6 ± 18.8) | ND |
| Ginkgolide B | 5.5 ± 4.5 (65.4 ± 38.9) | 9.2 ± 2.2 (86.6 ± 21.1) | 12.3 ± 1.8 (116.0 ± 16.7) | ND | ND |
| PCS | 0.3 ± 0.1 (1.5 ± 0.3) | 0.6 ± 0.15 (3.1 ± 0.8) | 0.5 ± 0.04 (2.3 ± 0.2) | 1.5 ± 0.8 (7.4 ± 3.8) | 17.6 ± 5.6 |
| PAF | - | - | - | - | 0.009 ± 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoupras, A.; Lordan, R.; Harrington, J.; Pienaar, R.; Devaney, K.; Heaney, S.; Koidis, A.; Zabetakis, I. The Effects of Oxidation on the Antithrombotic Properties of Tea Lipids against PAF, Thrombin, Collagen, and ADP. Foods 2020, 9, 385. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9040385
Tsoupras A, Lordan R, Harrington J, Pienaar R, Devaney K, Heaney S, Koidis A, Zabetakis I. The Effects of Oxidation on the Antithrombotic Properties of Tea Lipids against PAF, Thrombin, Collagen, and ADP. Foods. 2020; 9(4):385. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9040385
Chicago/Turabian StyleTsoupras, Alexandros, Ronan Lordan, Jack Harrington, Rebecca Pienaar, Karen Devaney, Stephanie Heaney, Anastasios Koidis, and Ioannis Zabetakis. 2020. "The Effects of Oxidation on the Antithrombotic Properties of Tea Lipids against PAF, Thrombin, Collagen, and ADP" Foods 9, no. 4: 385. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9040385