Setup of an Extraction Method for the Analysis of Carotenoids in Microgreens
Abstract
:1. Introduction
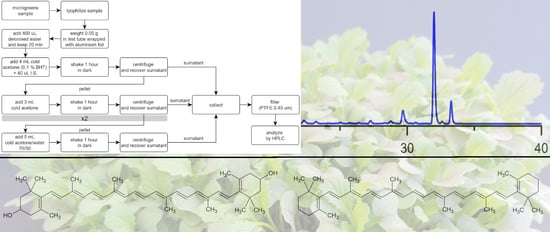
2. Materials and Methods
2.1. Materials and Reagents
2.2. Microgreens Production and Storage
2.3. Sample Pre-Treatments
2.4. Protection against Degradation
2.5. Optimization of the Carotenoids’ Extraction
- i.
- Evaluation of the solvent polarity mixing acetone with varying amounts of water (acetone 70%, 80%, 90%, 100%);
- ii.
- Evaluation of different extraction times (30 s, 10 min, 1, 5, 24 h);
- iii.
- Evaluation of the solvent/sample ratio (4, 5, 6, 12 mL of solvent per 0.05 g of sample);
- iv.
- Evaluation of repeated extractions.
2.6. HPLC Analysis of Carotenoids
2.7. Method Validity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Optimization of the Carotenoids’ Extraction
3.1.1. Effect of Solvent Polarity and Extraction Time
3.1.2. Effect of Solvent/Sample Ratio
3.1.3. Repeated Extractions
3.1.4. Method Validity
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Renna, M.; Di Gioia, F.; Leoni, B.; Mininni, C.; Santamaria, P. Culinary Assessment of Self-Produced Microgreens as Basic Ingredients in Sweet and Savory Dishes. J. Culin. Sci. Technol. 2017, 15, 126–142. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Castellino, M.; Renna, M.; Gattullo, C.E.; Calasso, M.; Terzano, R.; Allegretta, I.; Leoni, B.; Caponio, F.; Santamaria, P. Nutritional characterization and shelf-life of packaged microgreens. Food Funct. 2018, 9, 5629–5640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renna, M.; Castellino, M.; Leoni, B.; Paradiso, V.; Santamaria, P. Microgreens Production with Low Potassium Content for Patients with Impaired Kidney Function. Nutrients 2018, 10, 675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradiso, V.M.; Castellino, M.; Renna, M.; Leoni, B.; Caponio, F.; Santamaria, P. Simple tools for monitoring chlorophyll in broccoli raab and radish microgreens on their growing medium during cold storage. Prog. Nutr. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- De la Fuente, B.; López-García, G.; Mañez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the Bioaccessibility of Antioxidant Bioactive Compounds and Minerals of Four Genotypes of Brassicaceae Microgreens. Foods 2019, 8, 250. [Google Scholar] [CrossRef] [Green Version]
- Choe, U.; Yu, L.L.; Wang, T.T.Y. The Science behind Microgreens as an Exciting New Food for the 21st Century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; De Pascale, S.; Kyratzis, A.; Rouphael, Y. Microgreens as a Component of Space Life Support Systems: A Cornucopia of Functional Food. Front. Plant Sci. 2017, 8, 8–11. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Microgreens: Production, shelf life, and bioactive components. Crit. Rev. Food Sci. Nutr. 2017, 57, 2730–2736. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Viršilė, A.; Jankauskienė, J.; Sakalauskienė, S.; Duchovskis, P. Red Light-Dose or Wavelength-Dependent Photoresponse of Antioxidants in Herb Microgreens. PLoS ONE 2016, 11, e0163405. [Google Scholar] [CrossRef]
- Adadi, P.; Barakova, N.V.; Krivoshapkina, E.F. Selected Methods of Extracting Carotenoids, Characterization, and Health Concerns: A Review. J. Agric. Food Chem. 2018, 66, 5925–5947. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Barros, M.P.; Rodrigo, M.J.; Zacarias, L. Dietary carotenoid roles in redox homeostasis and human health. J. Agric. Food Chem. 2018, 66, 5733–5740. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M.; Rodriguez-Amaya, D.B. A scheme for obtaining standards and HPLC quantification of leafy vegetable carotenoids. Food Chem. 2002, 78, 389–398. [Google Scholar] [CrossRef]
- Niizu, P.Y.; Rodriguez-Amaya, D.B. New data on the carotenoid composition of raw salad vegetables. J. Food Compos. Anal. 2005, 18, 739–749. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Park, E.; Saftner, R.A.; Luo, Y.; Wang, Q. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: Microgreens. Postharvest Biol. Technol. 2015, 110, 140–148. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P. Blue light dosage affects carotenoids and tocopherols in microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Pantanizopoulos, N.I.; Sams, C.E.; Kopsell, D.E. Shoot tissue pigment levels increase in “Florida Broadleaf” mustard (Brassica juncea L.) microgreens following high light treatment. Sci. Hortic. 2012, 140, 96–99. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Xie, Z.; Yu, L.; Wang, Q. Effect of light exposure on sensorial quality, concentrations of bioactive compounds and antioxidant capacity of radish microgreens during low temperature storage. Food Chem. 2014, 151, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Sakalauskienė, S.; Samuolienė, G.; Jankauskienė, J.; Viršilė, A.; Novičkovas, A.; Sirtautas, R.; Miliauskienė, J.; Vaštakaitė, V.; Dabašinskas, L.; et al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Functional quality in novel food sources: Genotypic variation in the nutritive and phytochemical composition of thirteen microgreens species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. Some considerations in generating carotenoid data for food composition tables. J. Food Compos. Anal. 2000, 13, 641–647. [Google Scholar] [CrossRef]
- Kopec, R.E.; Cooperstone, J.L.; Cichon, M.J.; Schwartz, S.J. Analysis Methods of Carotenoids. In Analysis of Antioxidant-Rich Phytochemicals; Wiley-Blackwell: Oxford, UK, 2012; pp. 105–148. ISBN 9780813823911. [Google Scholar]
- Mertz, C.; Brat, P.; Caris-Veyrat, C.; Gunata, Z. Characterization and thermal lability of carotenoids and vitamin C of tamarillo fruit (Solanum betaceum Cav.). Food Chem. 2010, 119, 653–659. [Google Scholar] [CrossRef]
- Amorim-Carrilho, K.T.; Cepeda, A.; Fente, C.; Regal, P. Review of methods for analysis of carotenoids. TrAC Trends Anal. Chem. 2014, 56, 49–73. [Google Scholar] [CrossRef]
- Kimura, M.; Rodriguez-Amaya, D.B.; Godoy, H.T. Assessment of the saponification step in the quantitative determination of carotenoids and provitamins A. Food Chem. 1990, 35, 187–195. [Google Scholar] [CrossRef]
- Rasmussen, H.M.; Muzhingi, T.; Eggert, E.M.R.; Johnson, E.J. Lutein, zeaxanthin, meso-zeaxanthin content in egg yolk and their absence in fish and seafood. J. Food Compos. Anal. 2012, 27, 139–144. [Google Scholar] [CrossRef]
- Burns, D.T.; Danzer, K.; Townshend, A. Use of the Terms “Recovery” and “Apparent Recovery” in Analytical Procedures. Pure Appl. Chem. 2002, 74, 2201–2205. [Google Scholar] [CrossRef]
- Guideline, I.H.T. Validation of analytical procedures: Text and methodology Q2 (R1). In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, November 2005. [Google Scholar]
- Edelenbos, M.; Christensen, L.P.; Grevsen, K. HPLC Determination of Chlorophyll and Carotenoid Pigments in Processed Green Pea Cultivars (Pisum sativum L.). J. Agric. Food Chem. 2001, 49, 4768–4774. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef] [Green Version]








| Internal Standard | Violaxanthin | Lutein | β-Carotene | |
|---|---|---|---|---|
| Recovery | 97.2% | |||
| Linearity (Adjusted R2) | 0.999 | 0.999 | 0.999 | |
| Limit of detection (LOD) | 1.6 μg g−1 | 11.3 μg g−1 | ||
| Limit of quantitation (LOQ) | 5.2 μg g−1 | 15.9 μg g−1 | ||
| Experimental limit of quantitation (ELOQ) | 8.4 ± 0.5 μg g−1 | 9.1 ± 1.1 μg g−1 | ||
| Intra-day repeatability (C.V.%) | 4.4% | 5.7% | 6.9% | |
| Inter-day repeatability (C.V.%) | 4.1% | 4.8% | 5.3% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paradiso, V.M.; Castellino, M.; Renna, M.; Santamaria, P.; Caponio, F. Setup of an Extraction Method for the Analysis of Carotenoids in Microgreens. Foods 2020, 9, 459. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9040459
Paradiso VM, Castellino M, Renna M, Santamaria P, Caponio F. Setup of an Extraction Method for the Analysis of Carotenoids in Microgreens. Foods. 2020; 9(4):459. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9040459
Chicago/Turabian StyleParadiso, Vito Michele, Maria Castellino, Massimiliano Renna, Pietro Santamaria, and Francesco Caponio. 2020. "Setup of an Extraction Method for the Analysis of Carotenoids in Microgreens" Foods 9, no. 4: 459. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9040459