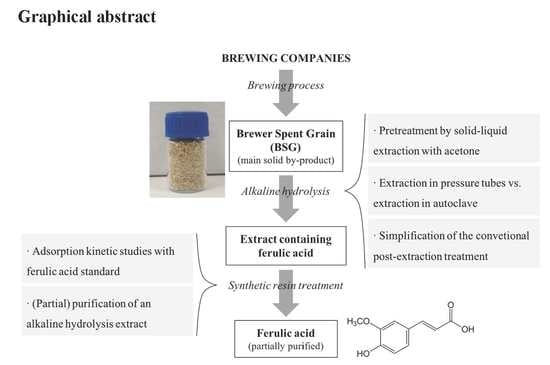
A Novel and Simpler Alkaline Hydrolysis Methodology for Extraction of Ferulic Acid from Brewer’s Spent Grain and its (Partial) Purification through Adsorption in a Synthetic Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Raw Material
2.3. Physicochemical Characterization
2.4. Alkaline Hydrolysis—Basic Procedure
2.5. Pretreatment by Solid–Liquid Extraction with Acetone
2.6. Extraction by Alkaline Hydrolysis in Autoclave
2.7. Simplification of the Procedure
2.8. Analysis of Extracts
2.8.1. Total Soluble Solids (TSS) Determination
2.8.2. Total Phenolic Content (TPC) Determination
2.8.3. Quantification of Ferulic Acid
2.8.4. Statistical Analysis
2.9. Purification by Adsorption on a Synthetic Resin
2.9.1. Kinetic Studies with FA Standard
2.9.2. Purification of an Alkaline Hydrolysis Extract
3. Results and Discussion
3.1. Physicochemical Characterization of BSG
3.2. Optimization of Alkaline Hydrolysis Conditions
3.3. Pretreatment by Solid–Liquid Extraction with Acetone
3.4. Extraction by Alkaline Hydrolysis in Autoclave
3.5. Simplification of the Procedure
3.6. Purification by Adsorption on a Synthetic Resin
3.6.1. Kinetic Studies with FA Standard
3.6.2. Purification of an Alkaline Hydrolysis Extract
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Gupta, M.; Abu-Ghannam, N.; Gallaghar, E. Barley for brewing: Characteristic changes during malting, brewing and applications of its by-products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 318–328. [Google Scholar] [CrossRef] [Green Version]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliyu, S.; Bala, M. Brewer’s spent grain: A review of its potentials and applications. Afr. J. Biotechnol. 2011, 10, 324–331. [Google Scholar] [CrossRef]
- McCarthy, A.L.; O’Callaghan, Y.C.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. Brewers’ spent grain; bioactivity of phenolic component, its role in animal nutrition and potential for incorporation in functional foods: A review. Proc. Nutr. Soc. 2013, 72, 117–125. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, A.; O’Callaghan, Y.C.; Neugart, S.; Piggott, C.O.; Connolly, A.; Jansen, M.A.; Krumbein, A.; Schreiner, M.; Fitzgerald, R.J.; O’Brien, N.M. The hydroxycinnamic acid content of barley and brewers’ spent grain (BSG) and the potential to incorporate phenolic extracts of BSG as antioxidants into fruit beverages. Food Chem. 2013, 141, 2567–2574. [Google Scholar] [CrossRef]
- Kaur, V.I.; Saxena, P.K. Incorporation of brewery waste in supplementary feed and its impact on growth in some carps. Bioresour. Technol. 2004, 91, 101–104. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Esteves, M.P.; Parajó, J.C.; Pereira, H.; Gírio, F.M. Production of oligosaccharides by autohydrolysis of brewery’s spent grain. Bioresour. Technol. 2004, 91, 93–100. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Acid hydrolysis and fermentation of brewer’s spent grain to produce xylitol. J. Sci. Food Agric. 2005, 85, 2453–2460. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Medeiros, R.; Gírio, F.M. Xylitol production by Debaryomyces hansenii in brewery spent grain dilute-acid hydrolysate: Effect of supplementation. Biotechnol. Lett. 2007, 29, 1887–1891. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Ferulic and p-coumaric acids extraction by alkaline hydrolysis of brewer’s spent grain. Ind. Crop. Prod. 2007, 25, 231–237. [Google Scholar] [CrossRef]
- Bartolomé, B.; Gómez-Cordovés, C.; Sancho, A.; Diez, N.; Ferreira, P.; Soliveri, J.; Copa-Patiño, J.L. Growth and release of hydroxycinnamic acids from Brewer’s spent grain by Streptomyces avermitilis CECT 3339. Enzym. Microb. Technol. 2003, 32, 140–144. [Google Scholar] [CrossRef]
- Moreira, M.M.; Morais, S.; Barros, A.; Delerue-Matos, C.; Guido, L.F. A novel application of microwave-assisted extraction of polyphenols from brewer’s spent grain with HPLC-DAD-MS analysis. Anal. Bioanal. Chem. 2012, 403, 1019–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, S.; Kwok, K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Priefert, H.; Rabenhorst, J.; Steinbüchel, A. Biotechnological production of vanillin. Appl. Microbiol. Biotechnol. 2001, 56, 296–314. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, M.; Rein, D.; Satue, M.T.; Huang, S.; German, J.B.; Frankel, E.N. Effect of Protein on the Antioxidant Activity of Phenolic Compounds in a Lecithin-Liposome Oxidation System. J. Agric. Food Chem. 1998, 8561, 917–922. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Buranov, A.U.; Mazza, G. Extraction and purification of ferulic acid from flax shives, wheat and corn bran by alkaline hydrolysis and pressurised solvents. Food Chem. 2009, 115, 1542–1548. [Google Scholar] [CrossRef]
- Ferri, M.; Happel, A.; Zanaroli, G.; Bertolini, M.; Chiesa, S.; Commisso, M.; Tassoni, A. Advances in combined enzymatic extraction of ferulic acid from wheat bran. New Biotechnol. 2020, 56, 38–45. [Google Scholar] [CrossRef]
- Long, L.; Ding, D.; Han, Z.; Zhao, H.; Lin, Q.; Ding, S. Thermotolerant hemicellulolytic and cellulolytic enzymes from Eupenicillium parvum 4-14 display high efficiency upon release of ferulic acid from wheat bran. J. Appl. Microbiol. 2016, 121, 422–434. [Google Scholar] [CrossRef]
- Moreira, M.M.; Morais, S.; Carvalho, D.O.; Barros, A.A.; Delerue-Matos, C.; Guido Luís, F. Brewer’s spent grain from different types of malt: Evaluation of the antioxidant activity and identification of the major phenolic compounds. Food Res. Int. 2013, 54, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.H.; Juang, R.S. Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: A review. J. Environ. Manag. 2009, 90, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Dávila-Guzman, N.E.; Cerino-Córdova, F.J.; Diaz-Flores, P.E.; Rangel-Mendez, J.R.; Sánchez-González, M.N.; Soto-Regalado, E. Equilibrium and kinetic studies of ferulic acid adsorption by Amberlite XAD-16. Chem. Eng. J. 2012, 183, 112–116. [Google Scholar] [CrossRef]
- Conidi, C.; Rodriguez-Lopez, A.; Garcia-Castello, E.M.; Cassano, A. Purification of artichoke polyphenols by using membrane filtration and polymeric resins. Sep. Purif. Technol. 2015, 144, 153–161. [Google Scholar] [CrossRef]
- Javanmardi, J.; Kubota, C. Variation of lycopene, antioxidant activity, total soluble solids and weight loss of tomato during postharvest storage. Postharvest Boil. Technol. 2006, 41, 151–155. [Google Scholar] [CrossRef]
- Gouveia, S.; Castilho, P.C. Antioxidant potential of Artemisia argentea L’Hér alcoholic extract and its relation with the phenolic composition. Food Res. Int. 2011, 44, 1620–1631. [Google Scholar] [CrossRef]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef] [Green Version]




| Assay | Test | [FA] (g/L) | Resin wt. (g) | Temperature |
|---|---|---|---|---|
| 1 | AdsA | 1 | 0.5 | Room temp. |
| AdsB | 0.5 | 0.25 | ||
| AdsC | 0.25 | 0.125 | ||
| 2 | AdsHC | 1 | 0.5 | Room temp. |
| AdsLC | 0.25 | 0.5 | ||
| 3 | AdsRT | 0.25 | 0.5 | Room temp. |
| AdsT6 | 0.25 | 0.5 | 6 °C |
| Particle Size (mm) | Percentage (%) | ||
|---|---|---|---|
| >1 | 3.83 | ± | 0.37 |
| 1–0.5 | 36.00 | ± | 1.48 |
| 0.5–0.25 | 41.06 | ± | 2.44 |
| 0.25–0.125 | 12.57 | ± | 2.65 |
| ≤0.125 | 6.76 | ± | 1.12 |
| Temp. (°C) | Time (Hours) | NaOH (%) | FA Yield (mg FA/100 g) | ||
|---|---|---|---|---|---|
| 60 | 1 | 2 | 46.95 | ± | 9.72 |
| 80 | 1 | 2 | 81.52 | ± | 17.89 |
| 100 | 1 | 2 | 214.18 | ± | 30.43 |
| 120 | 1 | 2 | 234.98 | ± | 8.03 |
| 100 | 1 | 2 | 214.67 | ± | 4.70 |
| 100 | 1.5 | 2 | 223.97 | ± | 6.82 |
| 100 | 2 | 2 | 223.05 | ± | 1.47 |
| 100 | 2.5 | 2 | 202.62 | ± | 19.65 |
| 100 | 3 | 2 | 223.09 | ± | 17.85 |
| 100 | 1 | 0.5 | 174.76 | ± | 23.64 |
| 100 | 1 | 1 | 199.63 | ± | 11.21 |
| 100 | 1 | 1.5 | 214.53 | ± | 23.26 |
| 100 | 1 | 2 | 204.44 | ± | 9.64 |
| 100 | 1 | 2.5 | 200.10 | ± | 22.07 |
| Not Pretreated | Pretreated | |||||
|---|---|---|---|---|---|---|
| TSS mg SE/100 g | 94.50 | ± | 8.39 a | 69.39 | ± | 3.28 b |
| TPC g GAE/100 g | 1010.44 | ± | 1.58 a | 1323.24 | ± | 143.30 b |
| FA yield mg FA/100 g | 259.21 | ± | 35.95 a | 270.32 | ± | 65.86 a |
| Ace Pressure Tubes | Autoclave | |||||
|---|---|---|---|---|---|---|
| TSS mg SE/100 g | 81.45 | ± | 2.59 a | 82.44 | ± | 9.17 a |
| TPC g GAE/100 g | 1194.20 | ± | 21.34 a | 1439.73 | ± | 102.02 b |
| FA yield mg FA/100 g | 203.41 | ± | 5.37 a | 280.61 | ± | 5.77 b |
| Normal Procedure | Simplified Procedure | |||||
|---|---|---|---|---|---|---|
| TSS mg SE/100 g | 94.50 | ± | 8.39 a | 123.70 | ± | 1.47 b |
| TPC g GAE/100 g | 1483.72 | ± | 90.03 a | 3342.86 | ± | 71.21 b |
| FA yield mg FA/100 g | 259.21 | ± | 35.95 a | 476.99 | ± | 25.94 b |
| Assay | Test | Parameter | SSD | ||||
|---|---|---|---|---|---|---|---|
| α | β | γ | δ | θ | |||
| 1 | AdsA | 0.2144 | 0.4721 | 18.1123 | 0.3122 | 1.6490 | 0.0006 |
| AdsB | 0.3169 | 0.3620 | 24.1647 | 0.3031 | 7.9226 | 0.0014 | |
| AdsC | 0.4262 | 0.4271 | 27.1620 | 0.1391 | 4.1121 | 0.0022 | |
| 2 | AdsHC | 0.2144 | 0.4721 | 18.1123 | 0.3122 | 1.6490 | 0.0006 |
| AdsLC | 0.1343 | 0.3759 | 15.7397 | 0.4861 | 2.2909 | 0.0014 | |
| 3 | AdsRT | 0.1343 | 0.3759 | 15.7401 | 0.4861 | 2.2910 | 0.0014 |
| AdsT6 | 0.1335 | 0.3992 | 24.5310 | 0.4604 | 3.4751 | 0.0013 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ideia, P.; Sousa-Ferreira, I.; Castilho, P.C. A Novel and Simpler Alkaline Hydrolysis Methodology for Extraction of Ferulic Acid from Brewer’s Spent Grain and its (Partial) Purification through Adsorption in a Synthetic Resin. Foods 2020, 9, 600. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9050600
Ideia P, Sousa-Ferreira I, Castilho PC. A Novel and Simpler Alkaline Hydrolysis Methodology for Extraction of Ferulic Acid from Brewer’s Spent Grain and its (Partial) Purification through Adsorption in a Synthetic Resin. Foods. 2020; 9(5):600. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9050600
Chicago/Turabian StyleIdeia, Pedro, Ivo Sousa-Ferreira, and Paula C. Castilho. 2020. "A Novel and Simpler Alkaline Hydrolysis Methodology for Extraction of Ferulic Acid from Brewer’s Spent Grain and its (Partial) Purification through Adsorption in a Synthetic Resin" Foods 9, no. 5: 600. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9050600