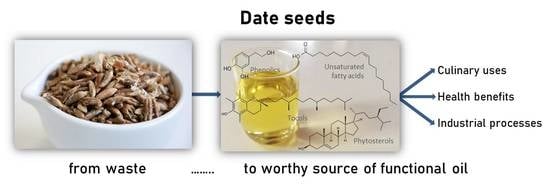
Date Seeds: A Promising Source of Oil with Functional Properties
Abstract
:1. Introduction
2. Chemical Composition of Date Seed Oil
2.1. Fatty Acid Composition
2.2. Tocopherol and Tocotrienol Compositions
2.3. Sterol Composition
2.4. Phenolic Compounds
3. Physicochemical Characteristics of Date Seed Oil
4. Date Seed Oil Extraction
4.1. Conventional Extraction: Soxhlet Extraction
4.2. Innovative Extraction Methods
4.2.1. Supercritical Fluid Extraction (SFE)
4.2.2. Ultrasonic-Assisted Extraction (UAE)
5. Potential Applications of Date Seed Oil
5.1. Culinary Uses
5.2. Health Beneficial Effects
5.3. Feedstock for Industrial Processes
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QC (accessed on 14 April 2020).
- Chandrasekaran, M.; Bahkali, A. Valorization of date palm (Phoenix dactylifera) fruit processing by-products and wastes using bioprocess technology-Review. Saudi J. Boil. Sci. 2013, 20, 105–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.; Kasapis, S.; Al-Kharusi, N.; Al-Marhubi, I.; Khan, A. Composition characterisation and thermal transition of date pits powders. J. Food Eng. 2007, 80, 1–10. [Google Scholar] [CrossRef]
- Mrabet, A.; Rodríguez-Gutiérrez, G.; Guillén-Bejarano, R.; Arcos, R.R.; Ferchichi, A.; Sindic, M.; Jiménez-Araujo, A. Valorization of Tunisian secondary date varieties (Phoenix dactylifera L.) by hydrothermal treatments: New fiber concentrates with antioxidant properties. LWT 2015, 60, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Ekpa, O.D.; Ebana, R.U.B. Comparative Studies of Manyanga, Palm and Coconut Oils: Anti-microbial effects of the oils and their metallic soaps on some bacteria and fungi. Global. J Pure Appl. Sci. 1996, 1, 155–163. [Google Scholar]
- Besbes, S.; Blecker, C.; Deroanne, C.; Drira, N.; Attia, H. Date seeds: Chemical composition and characteristic profiles of the lipid fraction. Food Chem. 2004, 84, 577–584. [Google Scholar] [CrossRef]
- Besbes, S.; Blecker, C.; Deroanne, C.; Lognay, G.; Drira, N.; Attia, H. Heating effects on some quality characteristics of date seed oil. Food Chem. 2005, 91, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Habib, H.; Ibrahim, W. Nutritional quality evaluation of eighteen date pit varieties. Int. J. Food Sci. Nutr. 2009, 60, 99–111. [Google Scholar] [CrossRef]
- Nehdi, I.; Omri, S.; Khalil, M.; Al-Resayes, S. Characteristics and chemical composition of date palm (Phoenix canariensis) seeds and seed oil. Ind. Crop. Prod. 2010, 32, 360–365. [Google Scholar] [CrossRef]
- Habib, H.; Kamal, H.; Ibrahim, W.; Al Dhaheri, A.S. Carotenoids, fat soluble vitamins and fatty acid profiles of 18 varieties of date seed oil. Ind. Crop. Prod. 2013, 42, 567–572. [Google Scholar] [CrossRef]
- Nehdi, I.; Sbihi, H.M.; Tan, C.; Rashid, U.; Al-Resayes, S.I. Chemical Composition of Date Palm (Phoenix dactylifera L.) Seed Oil from Six Saudi Arabian Cultivars. J. Food Sci. 2018, 83, 624–630. [Google Scholar] [CrossRef]
- Reddy, M.K.; Rani, H.D.; Deepika, C.N.; Samrawat, S.; Akshara, V.; Rajesh, K. Study on Physico-chemical Properties of Oil and Powder of Date Palm Seeds (Phoenix dactylifera). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 486–492. [Google Scholar] [CrossRef]
- Boukouada, M.; Yousfi, M. Phytochemical study of date seeds lipids of three fruits (Phoenix dactylifera L.) produced in Ouargla region. Ann. Fac. Sci. Sci. de l’Ingénieur 2009, 1, 66–74. [Google Scholar]
- Al-Shahib, W.; Marshall, R.J. Fatty acid content of the seeds from 14 varieties of date palm Phoenix dactylifera L. Int. J. Food Sci. Technol. 2003, 38, 709–712. [Google Scholar] [CrossRef]
- Bouallegue, K.; Allaf, T.; Besombes, C.; Ben Younes, R.; Allaf, K. Phenomenological modeling and intensification of texturing/grinding-assisted solvent oil extraction: Case of date seeds (Phoenix dactylifera L.). Arab. J. Chem. 2019, 12, 2398–2410. [Google Scholar] [CrossRef] [Green Version]
- Biglar, M.; Khanavi, M.; Hajimahmoodi, M.; Hassani, S.; Moghaddam, G.; Sadeghi, N.; Oveisi, M.R. Tocopherol Content and Fatty acid Profile of Different Iranian Date Seed Oils. Iran. J. Pharm. Res. IJPR 2012, 11, 873–878. [Google Scholar]
- Abdul Afiq, M.J.; Abdul Rahman, R.; Che Man, Y.B.; Al- Kahtani, H.A.; Mansor, T.S.T. Date seed and date seed oil. Int. Food Res. J. 2013, 20, 2035–2043. [Google Scholar]
- Al-Hooti, S.; Sidhu, J.S.; Qabazard, H. Physicochemical characteristics of five date fruit cultivars grown in the United Arab Emirates. Plant Foods Hum. Nutr. 1997, 50, 101–113. [Google Scholar] [CrossRef]
- Dehdivan, N.S.; Panahi, B. Physicochemical Properties of Seeds and Seeds Oil Extracted from Iranian Date Palm Cultivars. Biol. Forum An Int. J. 2017, 9, 139–144. [Google Scholar]
- Suresh, S.; Guizani, N.; Al-Ruzeiki, M.; Al-Hadhrami, A.; Al-Dohani, H.; Al-Kindi, I.; Rahman, M.S. Thermal characteristics, chemical composition and polyphenol contents of date-pits powder. J. Food Eng. 2013, 119, 668–679. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Sharanabasappa, G.; Parmjyothi, S.; Seshagiri, M.; Moersel, J.-T.; Hassanien, M.F.R. Profile and levels of fatty acids and bioactive constituents in mahua butter from fruit-seeds of buttercup tree [Madhuca longifolia (Koenig)]. Eur. Food Res. Technol. 2005, 222, 710–718. [Google Scholar] [CrossRef]
- Babu, S.V.; Veeresh, B.; Patil, A.A.; Warke, Y. Lauric acid and myristic acid prevent testosterone induced prostatic hyperplasia in rats. Eur. J. Pharmacol. 2009, 22, 193–199. [Google Scholar] [CrossRef]
- De Roos, N.; Schouten, E.G.; Katan, M.B. Consumption of a solid fat rich in lauric acid results in a more favorable serum lipid profile in healthy men and women than consumption of a solid fat rich in trans-fatty acids. J. Nutr. 2001, 131, 242–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desbois, A. Potential applications of antimicrobial fatty acids in medicine, agriculture and other industries. Recent Pat. Anti-Infect. Drug Discov. 2012, 7, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Besbes, S.; Blecker, C.; Deroanne, C.; Bahloul, N.; Lognay, G.; Drira, N.; Attia, H. DATE SEED OIL: PHENOLIC, TOCOPHEROL AND STEROL PROFILES. J. Food Lipids 2004, 11, 251–265. [Google Scholar] [CrossRef]
- Basciny, A.M.M.; Al-Marzooq, M.A. Production of mayonnaise from date pit oil. Food Nut Sci. 2011, 2, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Raza, M.Q.; Arshad, M.U.; Arshad, M.S.; Anjum, F.M. Characterization of compositional and functional characteristics of date seeds and oil (Phoenix dactylifera L.) from three varieties. Int. J. Biosci. 2019, 15, 1–4. [Google Scholar] [CrossRef]
- Laghouiter, O.K.; Benalia, M.; Gourine, N.; Djeridane, A.; Bombarda, I.; Yousfi, M. Chemical characterization and in vitro antioxidant capacity of nine Algerian date palm cultivars (Phoenix dactylifera L.) seed oil. Mediterr. J. Nutr. Metab. 2018, 11, 103–117. [Google Scholar] [CrossRef] [Green Version]
- Akbari, M. Oil characteristics and fatty acid profile of seeds from three varieties of date palm (Phoenix dactylifera) cultivars in Bushehr-Iran. Afr. J. Biotechnol. 2012, 11, 12088–12093. [Google Scholar] [CrossRef]
- Bouhlali, E.D.T.; Alem, C.; Ennassir, J.; Benlyas, M.; Mbark, A.N.; Zegzouti, Y.F. Phytochemical compositions and antioxidant capacity of three date ( Phoenix dactylifera L.) seeds varieties grown in the South East Morocco. J. Saudi Soc. Agric. Sci. 2017, 16, 350–357. [Google Scholar] [CrossRef]
- Ali, S.S.; Kasoju, N.; Luthra, A.; Singh, A.; Sharanabasava, H.; Sahu, A.; Bora, U. Indian medicinal herbs as sources of antioxidants. Food Res. Int. 2008, 41, 1–15. [Google Scholar] [CrossRef]
- Nehdi, I.; Sbihi, H.; Tan, C.; Al-Resayes, S.I. Evaluation and characterisation of Citrullus colocynthis (L.) Schrad seed oil: Comparison with Helianthus annuus (sunflower) seed oil. Food Chem. 2013, 136, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.; Hwang, K.T.; Shin, M.K.; Lee, B.K.; Kim, S.K.; Kim, S.Y.; Lee, K.-T.; Zu Kim, S. Tocols in caneberry seed oils. Food Chem. 2008, 111, 687–690. [Google Scholar] [CrossRef]
- Watson, R.W.; Preedy, V.R. Tocotrienols Vitamin E beyond Tocopherols; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef] [Green Version]
- Gunstone, F.D. Vegetable Oils in Food Technology, Blackwell Publishing; CRC Press: Boca Raton, FL, USA, 2002; p. 501. [Google Scholar] [CrossRef]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, R.; Radhakrishnan, A.K. Tocotrienol research: Past into present. Nutr. Rev. 2012, 70, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Degreyt, W. Effect of physical refining on selected minor components in vegetable oils. PhD Thesis, Université des Sciences Appliquées de Gent, Ghent, Belgium, 1998. [Google Scholar] [CrossRef]
- Mokbli, S.; Nehdi, I.; Sbihi, H.M.; Tan, C.; Al-Resayes, S.I.; Rashid, U. Yucca aloifolia Seed Oil: A New Source of Bioactive Compounds. Waste Biomass-Valoriz. 2017, 9, 1087–1093. [Google Scholar] [CrossRef]
- Nehdi, I.; Mokbli, S.; Sbihi, H.; Tan, C.; Al-Resayes, S.I. Chamaerops humilis L. var.argenteaAndré Date Palm Seed Oil: A Potential Dietetic Plant Product. J. Food Sci. 2014, 79, C534–C539. [Google Scholar] [CrossRef]
- Fahad, A.J.; Mehmet, M.O.; Oladipupu, Q.A.; Omer, N.A.; Kashif, G.; Elfadil, E.B. Effect of date varieties on physico-chemical properties, fatty acid composition, tocopherol contents, and phenolic compounds of some date seed and oils. J. Food Process. Preserv. 2017, 42, 1–6. [Google Scholar] [CrossRef]
- Boukouada, M.; Ghiaba, Z.; Gourine, N.; Bombarda, I.; Saidi, M.; Yousfi, M. Chemical composition and antioxidant activity of seed oil of two Algerian date palm cultivars (Phoenix dactylifera). Nat. Prod. Commun. 2014, 9, 1777–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, K.; Knowlton, S. Frying quality and oxidative stability of high-oleic corn oils. J. Am. Oil Chem. Soc. 1997, 74, 1317–1322. [Google Scholar] [CrossRef]
- Lercker, G.; Rodriguez-Estrada, M. Chromatographic analysis of unsaponifiable compounds of olive oils and fat-containing foods. J. Chromatogr. A 2000, 881, 105–129. [Google Scholar] [CrossRef]
- Syed, M.S.; Imran, H.; Syed, D.A.G. Estimation of Sterols in Edible Fats and Oils. Pak. J. Nutr. 2003, 2, 178–181. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.; Tew, K. Phytosterols in the prevention of human pathologies. Biomed. Pharmacother. 2003, 57, 321–325. [Google Scholar] [CrossRef]
- Tasioula-Margari, M.; Okogeri, O. Isolation and Characterization of Virgin Olive Oil Phenolic Compounds by HPLC/UV and GC-MS. J. Food Sci. 2001, 66, 530–534. [Google Scholar] [CrossRef]
- Besbes, S.; Blecker, C.; Deroanne, C.; Lognay, G.; Drira, N.; Attia, H. Quality Characteristics and Oxidative Stability of Date Seed Oil During Storage. Food Sci. Technol. Int. 2004, 10, 333–338. [Google Scholar] [CrossRef]
- Mahmoud Abdalla, R.S.; Albasheer, A.A.; El-Hussein, A.R.M.; Gadkariem, E.A. Physico-chemical characteristics of date seed oil grown in Sudan. Am. J. Appl. Sci. 2012, 9, 993–999. [Google Scholar] [CrossRef] [Green Version]
- Akinhanmi, T.F.; Atasie, V.N.; Akuntokun, P.O. Chemical composition and physicochemical properties of cashew nut (Anacardiumoccidentale) oil and cashew nut shell liquid. J. Agric. Food Environ. Sc. 2008, 2, 1–10. [Google Scholar]
- Devshony, S.; Eteshola, E.; Shani, A. Characteristics and some potential applications of date palm (Phoenix dactylifera L.) seeds and seed oil. J. Am. Oil Chem. Soc. 1992, 69, 595–597. [Google Scholar] [CrossRef]
- Wu, H.; Shi, J.; Xue, S.; Kakuda, Y.; Wang, N.; Jiang, Y.; Ye, X.; Li, Y.; Subramanian, J. Essential oil extracted from peach (Prunus persica) kernel and its physicochemical and antioxidant properties. LWT 2011, 44, 2032–2039. [Google Scholar] [CrossRef]
- O’Brien, R.D. Fats and oils. In Formulation and Processing for Application, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2009; p. 680. [Google Scholar] [CrossRef]
- Guderjan, M.; Elez-Martinez, P.; Knorr, D. Application of pulsed electric fields at oil yield and content of functional food ingredients at the production of rapeseed oil. Innov. Food Sci. Emerg. Technol. 2007, 8, 55–62. [Google Scholar] [CrossRef]
- Pearson, D. Chemical Analysis of Foods, 7th ed.; AVI Publishing: Westport, CT, USA, 1976. [Google Scholar]
- Egbuonu, A.; Aguguesi, R.; Samuel, R.; Ojunkwu, O.; Onyenmeri, F.; Uzuegbu, U. Some Physicochemical Properties of the Petroleum Ether-Extracted Watermelon (Citrullus lanatus) Seed Oil. Asian J. Sci. Res. 2015, 8, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Talabi, J.Y.; Enujiugha, V.N. Physical and chemical evaluation of oils from 305 selected underutilized oilseeds. Pelagia Res. Libr. 2014, 5, 9–12. [Google Scholar]
- Borchani, C.; Besbes, S.; Blecker, C.; Attia, H. Chemical characteristics and oxidative stability of sesame seed, sesame paste, and olive oils. J. Agric. Sci. Technol. 2010, 12, 585–596. [Google Scholar]
- Boran, G.; Karaçam, H.; Boran, M. Changes in the quality of fish oils due to storage temperature and time. Food Chem. 2006, 98, 693–698. [Google Scholar] [CrossRef]
- AOAC. (Association of Analytical Chemist): Official method of analysis 13th Ed. William Horwitz. Ed. Washington. DC. Assoc. Off. Anal. Chem. 1990, 7, 56–132. [Google Scholar]
- Nehdi, I.; Sbihi, H.; Tan, C.; Zarrouk, H.; Khalil, M.I.; Al-Resayes, S.I. Characteristics, composition and thermal stability of Acacia senegal (L.) Willd. seed oil. Ind. Crop. Prod. 2012, 36, 54–58. [Google Scholar] [CrossRef]
- Akintayo, E.T.; Bayer, E. Characterisation and some possible uses of Plukenetia conophora and Adenopus breviflorus seeds and seed oils. Bioresour. Technol. 2002, 85, 95–97. [Google Scholar] [CrossRef]
- Falade, O.; Adekunle, A.S.; Aderogba, M.A.; Atanda, S.O.; Harwood, C.; Adewusi, S.R. Physicochemical properties, total phenol and tocopherol of someAcacia seed oils. J. Sci. Food Agric. 2007, 88, 263–268. [Google Scholar] [CrossRef]
- Akbar, E.; Yaakob, Z.; Kamarudin, S.K.; Ismail, M.; Salimon, J. Characteristic and composition of Jatropha curcas oil seed from Malaysia and its potential as biodiesel feedstock. Eur. J. Sci. Res. 2009, 29, 396–403. [Google Scholar]
- Herchi, W.; Kallel, H.; Boukhchina, S. Physicochemical properties and antioxidant activity of Tunisian date palm (Phoenix dactylifera L.) oil as affected by different extraction methods. Food Sci. Technol. 2014, 34, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Fine, F.; Vian, M.A.; Tixier, A.-S.F.; Carré, P.; Pages, X.; Chemat, F. Les agro-solvants pour l’extraction des huiles végétales issues de graines oléagineuses. OCL 2013, 20, A502. [Google Scholar] [CrossRef] [Green Version]
- Takadas, F.; Doker, O. Extraction method and solvent effect on safflower seed oil production. Chem. Process. Eng. Res. 2017, 51, 9–17. [Google Scholar]
- Moreno, A.O.; Dorantes, L.; Galíndez, J.; Guzmán, R.I.; Galindez-Mayer, J. Effect of Different Extraction Methods on Fatty Acids, Volatile Compounds, and Physical and Chemical Properties of Avocado (Persea americanaMill.) Oil. J. Agric. Food Chem. 2003, 51, 2216–2221. [Google Scholar] [CrossRef]
- Ali, M.A.; Al-Hattab, T.A.; Al-Hydary, I.A. Extraction of date palm seed oil (Phoenix dactylifera) by soxhlet apparatus. Int. J. Adv. Eng. Technol. 2015, 8, 261–271. [Google Scholar]
- Ben-Youssef, S.; Fakhfakh, J.; Breil, C.; Vian, M.A.; Chemat, F.; Allouche, N. Green extraction procedures of lipids from Tunisian date palm seeds. Ind. Crop. Prod. 2017, 108, 520–525. [Google Scholar] [CrossRef]
- Jadhav, A.; Holkar, C.; Goswami, A.D.; Pandit, A.B.; Pinjari, D.V. Acoustic Cavitation as a Novel Approach for Extraction of Oil from Waste Date Seeds. ACS Sustain. Chem. Eng. 2016, 4, 4256–4263. [Google Scholar] [CrossRef]
- Aris, N.A.; Norhuda, I.; Adeib, I.S. Extraction of Phoenix dactylifera (Mariami) seeds oil using supercritical carbon dioxide (SC-CO2). Int. J. Chem. Environ. Eng. 2013, 4, 32–37. [Google Scholar]
- Al-Sumri, A.; Al-Siyabi, N.; Al-Saadi, R.; Al-Rasbi, S.; Al-Dallal, A. Study on the Extraction of Date Palm Seed Oil using Soxhlet Apparatus. Int. J. Sci. Eng. Res. 2016, 7, 1266–1270. [Google Scholar]
- Louaer, M.; Zermane, A.; Larkeche, O.; Meniai, A.H. Experimental study and optimization of the extraction of Algerian date stones oil ( Phoenix dactylifera L.) using supercritical carbon dioxide. J. Food Process. Eng. 2019, 42, e13049. [Google Scholar] [CrossRef]
- Palaniappan, T. Evaluation and Optimization of Cranberry Seed Oil Extraction Methods. Ph.D. Thesis, Department of Bioresource Engineering, McGill University, Montreal, QC, Canada, 2012; p. 100. [Google Scholar]
- De Melo, M.; Silvestre, A.J.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- King, J. Modern Supercritical Fluid Technology for Food Applications. Annu. Rev. Food Sci. Technol. 2014, 5, 215–238. [Google Scholar] [CrossRef]
- Hegel, P.; Camy, S.; Destrac, P.; Condoret, J.-S. Influence of pretreatments for extraction of lipids from yeast by using supercritical carbon dioxide and ethanol as cosolvent. J. Supercrit. Fluids 2011, 58, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.-Z.; Wang, A.; Wei, W.; Liu, Y.; Shi, W.-H. Analysis of the operation conditions for supercritical fluid extraction of seed oil. Sep. Purif. Technol. 2005, 43, 163–167. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, K.A.; Mohamed, A.; Abdulamir, A.S.; Abas, H.A. A Review on Supercritical Fluid Extraction as New Analytical Method. Am. J. Biochem. Biotechnol. 2008, 4, 345–353. [Google Scholar] [CrossRef]
- Al-Rawi, S.S.; Ibrahim, A.H.; Majid, A.S.A.; Majid, A.M.A.; Ab Kadir, M.O. Comparison of yields and quality of nutmeg butter obtained by extraction of nutmeg rind by soxhlet and supercritical carbon dioxide (SC-CO2). J. Food Eng. 2013, 119, 595–601. [Google Scholar] [CrossRef]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Babaei, M.R.; Jabbari, A.; Yamini, Y. Solid–liquid extraction of fatty acids of some variety of Iranian rice in closed vessel in the absence and presence of ultrasonic waves. Asia J. Chem. 2006, 18, 57–64. [Google Scholar]
- Luque-García, J.; De Castro, M.D.L. Ultrasound-assisted Soxhlet extraction: An expeditive approach for solid sample treatment. J. Chromatogr. A 2004, 1034, 237–242. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef]
- Samaram, S.; Mirhosseini, H.; Tan, C.; Ghazali, H.M. Ultrasound-Assisted Extraction (UAE) and Solvent Extraction of Papaya Seed Oil: Yield, Fatty Acid Composition and Triacylglycerol Profile. Molecules 2013, 18, 12474–12487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanisavljević, I.T.; Lazić, M.; Veljković, V.B. Ultrasonic extraction of oil from tobacco (Nicotiana tabacum L.) seeds. Ultrason. Sonochem. 2007, 14, 646–652. [Google Scholar] [CrossRef]
- Khoei, M.; Chekin, F. The ultrasound-assisted aqueous extraction of rice bran oil. Food Chem. 2016, 194, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Samaram, S.; Mirhosseini, H.; Tan, C.; Ghazali, H.M.; Bordbar, S.; Serjouie, A. Optimisation of ultrasound-assisted extraction of oil from papaya seed by response surface methodology: Oil recovery, radical scavenging antioxidant activity, and oxidation stability. Food Chem. 2015, 172, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Böger, B.R.; Salviato, A.; Valezi, D.; Di Mauro, E.; Georgetti, S.R.; Kurozawa, L.E. Optimization of ultrasound-assisted extraction of grape-seed oil to enhance process yield and minimize free radical formation. J. Sci. Food Agric. 2018, 98, 5019–5026. [Google Scholar] [CrossRef]
- Chemat, F. High power ultrasound effects on lipid oxidation of refined sunflower oil. Ultrason. Sonochem. 2004, 11, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Ines, D.; Sonia, B.; Fatma, B.A.; Souhail, B.; Hamadi, A.; Hamida, T.; Basma, H. Date seed oil inhibits Hydrogen peroxide-induced oxidative stress in human epidermal keratinocytes. Int. J. Dermatol. 2010, 49, 262–268. [Google Scholar] [CrossRef]
- Ines, D.; Boudaya, S.; Ben Abdallah, F.; Turki, H.; Attia, H. Effect of Date Seed Oil on p53 Expression in Normal Human Skin. Connect. Tissue Res. 2010, 51, 55–58. [Google Scholar] [CrossRef]
- Lecheb, F.; Benamara, S. Feasability of a cosmetic cream added with aqueous extract and oil from date (Phoenix dactylifera L.) fruit seed using experimental design. J. Cosmet. Sci. 2015, 66, 359–370. [Google Scholar]
- Ben Abdallah, F.; Nozha, C.F.; Ines, D.; Hamadi, A.; Basma, H.; Leila, A.K. Sperm quality improvement after date seed oil in vitro supplementation in spontaneous and induced oxidative stress. Asian J. Androl. 2009, 11, 393–398. [Google Scholar] [CrossRef] [Green Version]
- Qadir, A.; Singh, S.P.; Akhtar, J.; Ali, A.; Arif, M. Chemical Composition of Saudi Arabian Sukkari variety of Date Seed Oil and Extracts Obtained by Slow Pyrolysis. Indian J. Pharm. Sci. 2018, 80, 940–946. [Google Scholar] [CrossRef]
- Larrucea, E. Combined effect of oleic acid and propylene glycol on the percutaneous penetration of tenoxicam and its retention in the skin. Eur. J. Pharm. Biopharm. 2001, 52, 113–119. [Google Scholar] [CrossRef]
- Azeem, M.; Hanif, M.A.; Al-Sabahi, J.N.; Khan, A.A.; Naz, S.; Ijaz, A. Production of biodiesel from low priced, renewable and abundant date seed oil. Renew. Energy 2016, 86, 124–132. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Taher, H.; Al Dhaheri, S.; Wajeeh, S.; Nour, M.; El-Najjar, E. Biodiesel Production from Oils Extracted from Date Pits. Green Sustain. Chem. 2017, 7, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Yousuf, R.; Winterburn, J. Waste date seed oil extract as an alternative feedstock for Poly(3-hydroxybutyrate) synthesis. Biochem. Eng. J. 2017, 127, 68–76. [Google Scholar] [CrossRef] [Green Version]
| References | Besbes et al. (2004) [25] | Basciny et al. (2011) [26] | Dehdivan et al. (2017) [19] | Nehdi et al. (2018) [11] | Raza et al. (2019) [27] | Laghouiter et al. (2018) [28] | Akbni et al. (2012) [29] | Bouhlali et al. (2017) [30] |
|---|---|---|---|---|---|---|---|---|
| Saturated | ||||||||
| Capric acid (10:0) | 0.80 | 0.25 | 0.2–0.5 | 0.71 | 0.48–0.55 | nd 1 | 0.47–0.50 | nd |
| Lauric acid (12:0) | 17.8 | 35.31 | 14–15.8 | 10.36 | 25.7–30.8 | 6.6–25.4 | 25.6–30.8 | 16.7–20.3 |
| Myristic acid (14:0) | 9.84 | 0.04 | 10.6–10.9 | 10.44 | 6.9–16.7 | 9.3–19.3 | 13.3–16.9 | 10.2–12.3 |
| Palmitic acid (16:0) | 10.9 | 12.58 | 10.8–11.8 | 12.83 | 11.9–13.1 | 9.6–17.6 | 11.9–13.1 | 9.8–10–9 |
| Stearic acid (18:0) | 5.67 | 3.3 | 3–3.4 | 5.56 | 1.8–2.3 | 0.8–3.4 | 1.8–2.3 | 2.9–3.7 |
| Monounsaturated | ||||||||
| Palmitoleic acid (16:1) | 0.11 | nd | 0.2–0.4 | nd | nd | 0.09–1.66 | nd | 0.05–0.09 |
| Oleic acid (18:1) | 41.3 | 39.5 | 48.1–50.5 | 51.45 | 31.5–37.6 | 37.8–52.5 | 31.5–37.6 | 44.9–48.4 |
| Gadoleic acid (20:1) | nd | nd | 0.2–0.4 | 0.37–0.52 | nd | 0.16–0.65 | nd | 0.26–0.40 |
| Polyunsaturated | ||||||||
| Linoleic acid (18.2) | 12.2 | 8.2 | 7.7–8.2 | 7.2 | 4.4–6.9 | 5.7–10.4 | 4.4–7.0 | 8.30–9.02 |
| Linolenic acid (18:3) | 1.68 | 0.81 | 0.4–0.7 | nd | nd | 0.10–0.59 | nd | 0.09–0.21 |
| Content | Composition | References | |
|---|---|---|---|
| Tocols (mg/100 g) | 32–74 | α-tocopherol (74%), (β+γ)-tocopherol (40.56%), δ-tocopherol (28.41%) | Laghouiter et al. (2018) [28] |
| 54.65 | α-tocotrienol (63.28%), γ-tocopherol (17.72%), γ-tocotrienol (11.84%), δ-tocotrienol (3.60%), α-tocopherol (1.85%), β-tocopherol (1.7%) | Fahad et al. (2017) [42] | |
| 0.053–0.143 | α-tocopherol (51.02%), (β+γ)-tocopherol (30.61%), δ-tocopherol (12.24%) | Boukaouada et al. (2014) [43] | |
| 1.01–1.86 | α-tocopherol (52.54%), α-tocopheryl acetate (27.68%), γ-tocopherol (19.76%) | Habib et al. (2013) [10] | |
| 24.97–42.08 | α-tocopherol (38.8%), γ-tocopherol (5.4%), δ-tocopherol (2.4%) | Besbes et al. (2004) [25] | |
| 51.54 | α-tocotrienol (66%), γ-tocopherol (10.3%), γ-tocotrienol (4.6%), δ-tocopherol (1%), β-tocopherol (0.9%), α-tocopherol (0.6%) | Nehdi et al. (2010) [9] | |
| 70.75 | α-tocotrienol (30.19%), γ-tocopherol (23.61%), γ-tocotrienol (19.07%), α-tocopherol (17.52%), δ-tocotrienol (5.89%), β-tocopherol (2.42%), δ-tocopherol (0.9%) | Nehdi et al. (2018) [11] | |
| Sterols (mg/100 g) | 300–350 | β-sitosterol (80%), campesterol (10%), Δ5-avenasterol (4.5%), stigmasterol (2.42%), cholesterol (0.96%), Δ5,24-stigmastadienol (0.41%) | Besbes et al. (2004) [25] |
| 470–845 | ND 1 | Laghouiter et al. (2018) [28] | |
| 336 | β-sitosterol (76%), campesterol (8.89%), Δ5-avenesterol (8.79%), Δ5,24-stigmastadienol (2.73%), Δ7-avenasterol (1.18%), stigmasterol (1.09%), Δ7-stigmastenol (0.79%), cholesterol (0.42%) | Nehdi et al. (2010) [9] | |
| Phenols (mg/kg) | 220.3–520.8 | Hydroxytyrosol (10.21%), protocatechuic acid (9.62%), tyrosol (8.10%), caffeic acid (4.95%), gallic acid (4.11%), p-coumaric acid (0.26%), oleuropein (0.18%) | Besbes et al. (2004) [25] |
| 640–1270 | ND | Boukaouada et al. (2014) [43] |
| Method | Conditions of Extraction | Conclusions | References |
|---|---|---|---|
| Soxhlet | Extraction yield 5% (chloroform, hexane), 4 h Extraction yield 1% (propanol, methanol), 4 h | The yield is better with non-polar solvents (n-hexane, chloroform) compared to polar solvents (methanol and propanol) | Ali et al. (2015) [70] |
| Soxhlet | Extraction yield 4.44%, hexane, 8 h | The ultrasound- and microwave-assisted extractions reduced the extraction time compared to Soxhlet and of extraction yield compared to maceration | Ben-Youssef et al. (2017) [71] |
| Ultrasound | Extraction yield 6.18%, hexane, 15 g, 20 °C, 30 min | ||
| Microwave | Extraction yield 4.74%, methyltetrahydrofuran (MeTHF), 15 g, 30 min | ||
| Maceration | Extraction yield 4.04%, MeTHF, 15 g, RT, 30 min | ||
| Soxhlet | Extraction yield 8.5%, hexane, 50 g, 78 °C, 3 h | The extraction by ultrasound shortened extraction time when compared to Soxhlet, reduced energy consumption, and had higher yield. | Jadhav et al. (2016) [72] |
| Maceration | Extraction yield 4.2%, hexane, 50 g, RT, 3 days | ||
| Ultrasound | Extraction yield 8.5% hexane, 50 g, 20 °C, 45 min | ||
| SC-CO2 | Extraction yield 3%, 5 g, 40 min, 70 °C | In this case, the oil yield was very low, probably due to several compatibilities of CO2 with the oil. | Aris et al. (2013) [73] |
| Soxhlet | Oil yield 8.5%, 120 min, hexane | The oil composition indicated the presence of low molecular weight saturated fatty acids. | Ali et al. (2015) [70] |
| Soxhlet | Oil yield 9.78, 8, and 9.5%, methanol, ethanol and acetone, respectively | The best conditions were found using methanol 15 °C above its boiling point, particle size range of 0.212–1 mm, 4 h. | Al-Sumri et al. (2016) [74] |
| SC-CO2 | Oil yield 14%, 250 bar, 333 K | The pressure and the interaction between the pressure and temperature had a positive significant effect on the extraction yield | Mehdi et al. (2019) [75] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrabet, A.; Jiménez-Araujo, A.; Guillén-Bejarano, R.; Rodríguez-Arcos, R.; Sindic, M. Date Seeds: A Promising Source of Oil with Functional Properties. Foods 2020, 9, 787. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9060787
Mrabet A, Jiménez-Araujo A, Guillén-Bejarano R, Rodríguez-Arcos R, Sindic M. Date Seeds: A Promising Source of Oil with Functional Properties. Foods. 2020; 9(6):787. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9060787
Chicago/Turabian StyleMrabet, Abdessalem, Ana Jiménez-Araujo, Rafael Guillén-Bejarano, Rocío Rodríguez-Arcos, and Marianne Sindic. 2020. "Date Seeds: A Promising Source of Oil with Functional Properties" Foods 9, no. 6: 787. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9060787