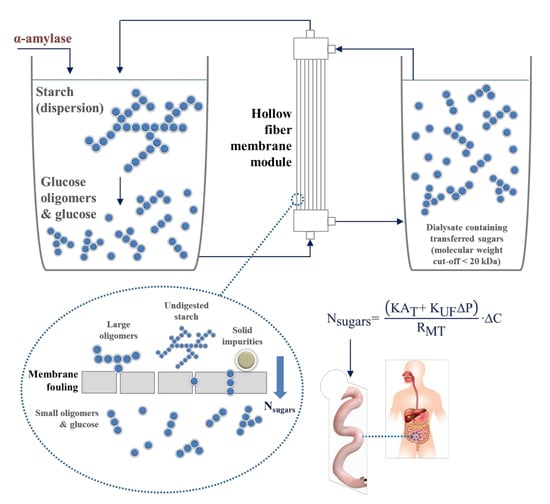
Simulation of Human Small Intestinal Digestion of Starch Using an In Vitro System Based on a Dialysis Membrane Process
Abstract
:1. Introduction
1.1. In Vitro Systems to Simulate Human Digestion
1.2. Kinetics of Starch Digestion
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gelatinized Starch Dispersions
2.3. In Vitro Starch Digestion
2.3.1. In Vitro Potato Starch Digestion Using the i-IDS
2.3.2. In Vitro Potato Starch Digestion Under Batch Conditions
2.3.3. Measurements of Reducing Sugars Concentration
2.4. Dialysis Membrane Process and Phenomenological Approach
2.5. Statistical Analysis of Data
3. Results and Discussion
3.1. Concentration of Reducing Sugars in the i-IDS under Different Operational Conditions
3.2. Mass of Reducing Sugars Obtained under Different Operating Conditions of the i-IDS and the In Vitro Batch Digestion of Starch
3.3. Fouling Analysis and Its Effect on Dialysis Performance
3.4. Scaling-Up to the Human Small Intestine
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| ΔP | Transmembrane pressure (Pa) |
| AT | Total area of mass transfer (m2) |
| Initial reducing sugars concentration (g/m3) | |
| Initial starch concentration in the bulk of the feed (g/m3) | |
| Concentration of reducing sugars in the bulk of the dialysate (g/m3) | |
| Concentration of reducing sugars in the bulk of the feed (g/m3) | |
| Concentration of starch in the bulk of the feed over time (g/m3) | |
| JC | Flux of reducing sugars transferred by convective effect (g/m2 min) |
| JD | Flux of reducing sugars transferred by diffusive effect (g/m2 min) |
| Jrs | Total flux of reducing sugars in the dialysis membrane process (g/m2 min) |
| K | Overall mass transfer coefficient (m/min) |
| KUF | Ultrafiltration coefficient (m3/Pa min) |
| k | Degradation rate constant (1/min) |
| M0 | Initial mass of potato starch (g) |
| MrsD | Dialysate reducing sugars mass (g) |
| MrsF | Feed reducing sugars mass (g) |
| MT | Maximum mass of reducing sugars generated from batch experiments (g) |
| Nrs | Overall mass transfer of reducing sugars transferred through the membrane (g/min) |
| RMT | Mass transfer resistance (dimensionless) |
| rs∝ | Maximum mass of reducing sugars (g) |
| rst | Mass of reducing sugars generated over time (g) |
| t | Time (min) |
| VF | Feed volume (m3) |
References
- Singh, J.; Dartois, A.; Kaur, L. Starch digestibility in food matrix: A review. Trends Food Sci. Tech. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Englyst, K.N.; Englyst, H.N.; Hudson, G.J.; Cole, T.J.; Cummings, J.H. Rapidly available glucose in foods: An in vitro measurement that reflects the glycemic response. Am. J. Clin. Nutr. 1999, 69, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Parada, J.; Aguilera, J.M. Review: Starch matrices and the glycemic response. Food Sci. Technol. Int. 2011, 17, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Etienne-Mesmin, L.; Livrelli, V.; Denis, S.; Blanquet-Diot, S.; Alric, M. Relevance and challenges in modeling human gastric and small intestinal digestion. Trends Biotechnol. 2012, 30, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.; Alric, M.; Blanquet-Diot, S.; Bornhorst, G.; Cueva, C.; Deglaire, A.; Denis, S.; Ferrua, M.; Havenaar, R.; Lelieveld, J.; et al. Can dynamic in vitro digestion systems mimic the physiological reality. Crit. Rev. Food Sci. 2019, 59, 1546–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, L.; Retamal, C.; Torres, H.; Zúñiga, R.N.; Troncoso, E. Development of an in vitro mechanical gastric system (IMGS) with realistic peristalsis to assess lipid digestibility. Food Res. Int. 2016, 90, 216–225. [Google Scholar] [CrossRef]
- Dahlgren, D.; Lennernäs, H. Intestinal permeability and drug absorption: Predictive experimental, computational and in vivo approaches. Pharmaceutics 2019, 11, 411. [Google Scholar] [CrossRef] [Green Version]
- Dahlgren, D.; Roos, C.; Sjögren, E.; Lennernäs, H. Direct in vivo human intestinal permeability (Peff) determined with different clinical perfusion and intubation methods. J. Pharm. Sci. 2015, 104, 2702–2726. [Google Scholar] [CrossRef] [Green Version]
- Petri, N.; Tannergren, C.; Holst, B.; Mellon, F.A.; Bao, Y.; Plumb, G.W.; Bacon, J.; O’Leary, K.A.; Kroon, P.A.; Knutson, L.; et al. Absorption/metabolism of sulforaphane and quercetin, and regulation of phase II enzymes, in human jejunum in vivo. Drug Metab. Dispos. 2003, 31, 805–813. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathi, S.; Bhushani, J.A.; Anandharamakrishnan, C. Engineered small intestinal system as an alternative to in-situ intestinal permeability model. J. Food Eng. 2018, 222, 110–114. [Google Scholar] [CrossRef]
- Li, W.; Zhang, T.; Ye, Y.; Zhang, X.; Wu, B. Enhanced bioavailability of tripterine through lipid nanoparticles using broccoli-derived lipids as a carrier material. Int. J. Pharm. 2015, 495, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Lennernäs, H.; Knutson, L.; Knutson, T.; Hussain, A.; Lesko, L.; Salmonson, T.; Amidon, G.L. The effect of amiloride on the in vivo effective permeability of amoxicillin in human jejunum: Experience from a regional perfusion technique. Eur. J. Pharm. Sci. 2002, 15, 271–277. [Google Scholar] [CrossRef]
- Sjögren, E.; Dahlgren, D.; Roos, C.; Lennernäs, H. Human in vivo regional intestinal permeability: Quantitation using site-specific drug absorption data. Mol. Pharm. 2015, 12, 2026–2039. [Google Scholar] [CrossRef] [PubMed]
- Hofsäss, M.A.; de Souza, J.; Silva-Barcellos, M.N.; Bellavinha, K.R.; Abrahamsson, B.; Cristofoletti, R.; Groot, D.W.; Parr, A.; Langguth, P.; Polli, J.E.; et al. Biowaiver monographs for immediate-release solid oral dosage forms: Folic acid. J. Pharm. Sci. 2017, 106, 3421–3430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjövall, J.; Alván, G.; Åkerlund, J.E.; Svensson, J.O.; Paintaud, G.; Nord, C.E.; Angelin, B. Dose-dependent absorption of amoxicillin in patients with an ileostomy. Eur. J. Clin. Pharmacol. 1992, 43, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Piena-Spoel, M.; Albers, M.J.I.J.; ten Kate, J.; Tibboel, D. Intestinal permeability in newborns with necrotizing enterocolitis and controls: Does the sugar absorption test provide guidelines for the time to re-introduce enteral nutrition. J. Pediatr. Surg. 2001, 36, 587–592. [Google Scholar] [CrossRef]
- McDougall, G.J.; Conner, S.; Pereira-Caro, G.; Gonzalez-Barrio, R.; Brown, E.M.; Verrall, S.; Stewart, D.; Moffet, T.; Ibars, M.; Lawther, R.; et al. Tracking (poly)phenol components from raspberries in ileal fluid. J. Agric. Food Chem. 2014, 62, 7631–7641. [Google Scholar] [CrossRef]
- Gim-Krumm, M.; Donoso, P.; Zuñiga, R.N.; Estay, H.; Troncoso, E. A comprehensive study of glucose transfer in the human small intestine using an in vitro intestinal digestion system (i-IDS) based on a dialysis membrane process. J. Membrane. Sci. 2018, 564, 700–711. [Google Scholar] [CrossRef]
- Magallanes-Cruz, P.A.; Flores-Silva, P.C.; Bello-Perez, L.A. Starch structure influences its digestibility: A review. J. Food Sci. 2017, 82, 2016–2023. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.-J.; Shin, D.-H.; Lim, S.-T. In vitro starch digestibility and estimated glycemic index of chemically modified corn starches. Food Res. Int. 2008, 41, 579–585. [Google Scholar] [CrossRef]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Ferrer-Mairal, A.; Peñalva-Lapuente, C.; Iglesia, I.; Urtasun, L.; De Miguel-Etayo, P.; Remón, S.; Cortés, E.; Moreno, L.A. In vitro and in vivo assessment of the glycemic index of bakery products: Influence of the reformulation of ingredients. Eur. J. Nutr. 2012, 51, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.C. Glycemic load and chronic disease. Nutr. Rev. 2003, 61 (Suppl. S5), S49–S55. [Google Scholar] [CrossRef]
- Kansy, M.; Senner, F.; Gubernator, K. Physicochemical high throughput screening: Parallel artificial membrane permeation assay in the description of passive absorption processes. J. Med. Chem. 1998, 41, 1007–1010. [Google Scholar] [CrossRef]
- Le Ferrec, E.; Chesne, C.; Artusson, P.; Brayden, D.; Fabre, G.; Gires, P.; Grillou, F.; Rousset, M.; Rubas, W.; Scarino, M.-L. In vitro models of the intestinal barrier: The report and recommendations of ECVAM workshop 461.2. Altern. Lab. Anim. 2001, 29, 649–668. [Google Scholar] [CrossRef]
- Blanquet, S.; Zeijdner, E.; Beyssac, E.; Meunier, J.; Denis, S.; Havenaar, R.; Alric, M. A dynamic artificial gastrointestinal system for studying the behavior of orally administered drug dosage forms under various physiological conditions. Pharm. Res. 2004, 21, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Tharakan, A.; Norton, I.T.; Fryer, P.J.; Bakalis, S. Mass transfer and nutrient absorption in a simulated model of small intestine. J. Food Sci. 2010, 75, E339–E346. [Google Scholar] [CrossRef] [PubMed]
- Gouseti, O.; Jaime-Fonseca, M.R.; Fryer, P.J.; Mills, C.; Wickham, M.S.J.; Bakalis, S. Hydrocolloids in human digestion: Dynamic in-vitro assessment of the effect of food formulation on mass transfer. Food Hydrocoll. 2014, 42, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Wright, N.D.; Kong, F.; Williams, B.S.; Fortner, L. A human duodenum model (HDM) to study transport and digestion of intestinal contents. J. Food Eng. 2016, 171, 129–136. [Google Scholar] [CrossRef]
- Broek, A.P.; Teunis, H.A.; Bargeman, D.; Sprengers, E.D.; Smolders, C.A. Characterization of hollow fiber hemodialysis membranes: Pore size distribution and performance. J. Membrane Sci. 1992, 73, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Tagaya, M.; Nagoshi, S.; Matsuda, M.; Takahashi, S.; Okano, S.; Hara, K. Hemodialysis membrane coated with a polymer having a hydrophilic blood-contacting layer can enhance diffusional performance. Int. J. Artif. Organs. 2017, 40, 665–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kee, C.M.; Idris, A. Permeability performance of different molecular weight cellulose acetate hemodialysis membrane. Sep. Purif. Technol. 2010, 75, 102–113. [Google Scholar] [CrossRef]
- Seita, Y.; Mochizuki, A.; Nak Agawa, M.; Takanashi, A.; Yamashita, S. Polyether-segmented nylon hemodialysis membranes. I. Preparation and permeability characteristics of polyether-segmented nylon 610 hemodialysis membrane. J. Appl. Polym. Sci. 1997, 69, 1703–1711. [Google Scholar] [CrossRef]
- Mochizuki, A.; Seita, Y.; Endo, F.; Nishi, T.; Saiga, N.; Yamashita, S. Polyether-segmented nylon hemodialysis membranes. II. Morphologies and permeability characteristics of polyether-segmented nylon 610 membrane prepared by the phase inversion method. J. Appl. Polym. Sci. 1997, 65, 1713–1721. [Google Scholar] [CrossRef]
- Sakai, K. Determination of pore size and pore size distribution: 2. Dialysis membranes. J. Membrane Sci. 1994, 96, 91–130. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Whistler, R.L. Starch: Chemistry and Technology, 3rd ed.; Elsevier: New York, NY, USA, 2009; pp. 237–292. [Google Scholar]
- Cummings, J.H.; Englyst, H.N. Gastrointestinal effects of food carbohydrate. Am. J. Clin. Nutr. 1995, 61, 938S–945S. [Google Scholar] [CrossRef]
- Warren, F.J.; Zhang, B.; Waltzer, G.; Gidley, M.J.; Dhital, S. The interplay of α-amylase and amyloglucosidase activities on the digestion of starch in in vitro enzymic systems. Carbohyd. Polym. 2015, 117, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Holm, J.; Lundquist, I.; Björck, I.; Eliasson, A.C.; Asp, N. Degree of starch gelatinization, digestion rate of starch in vitro, and metabolic response in rats. Am. J. Clin. Nutr. 1988, 47, 1010–1016. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch structure and digestibility enzyme-substrate relationship. World Poultry Sci. J. 2004, 60, 186–195. [Google Scholar] [CrossRef]
- Brand, J.C.; Nicholson, P.L.; Thorburn, A.W.; Truswell, A.S. Food processing and the glycemic index. Am. J. Clin. Nutr. 2006, 42, 1192–1196. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Hudson, G.J.; Cummings, J.H. Measurement of resistant starch in vitro and in vivo. Brit. J. Nutr. 1996, 75, 749–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butterworth, P.J.; Warren, F.J.; Grassby, T.; Patel, H.; Ellis, P.R. Analysis of starch amylolysis using plots for first-order kinetics. Carbohyd. Polym. 2012, 87, 2189–2197. [Google Scholar] [CrossRef]
- Patel, H.; Day, R.; Butterworth, P.J.; Ellis, P.R. A mechanistic approach to studies of the possible digestion of retrograded starch by α-amylase revealed using a log of slope (LOS) plot. Carbohyd. Polym. 2014, 113, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, C.H.; Maillot, M.; Parker, R.; Warren, F.J. A comparison of the kinetics of in vitro starch digestion in smooth and wrinkled peas by porcine pancreatic alpha-amylase. Food Chem. 2018, 244, 386–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dartois, A.; Singh, J.; Kaur, L.; Singh, H. Influence of guar gum on the in vitro starch digestibility-Rheological and microstructural characteristics. Food Biophys. 2010, 5, 149–160. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Cańtizares-Macías, P.; Hernández-Garciadiego, L.; Gómez-Ruíz, H. An automated flow injection analysis procedure for the determination of reducing sugars by DNSA method. J. Food Sci. 2001, 66, 407–411. [Google Scholar] [CrossRef]
- Avaltroni, F.; Bouquerand, P.E.; Normand, V. Maltodextrin molecular weight distribution influence on the glass transition temperature and viscosity in aqueous solutions. Carbohyd. Polym. 2004, 58, 323–334. [Google Scholar] [CrossRef]
- Bednarska, K.A. Kinetic Modelling of Enzymatic Starch Hydrolysis. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2015. [Google Scholar]
- Apar, D.K.; Özbek, B. Estimation of kinetic parameters for rice starch hydrolysis inhibited by added materials. Chem. Eng. Commun. 2007, 194, 334–344. [Google Scholar] [CrossRef]
- Pinelo, M.; Jonsson, G.; Meyer, A.S. Membrane technology for purification of enzymatically produced oligosaccharides: Molecular and operational features affecting performance. Sep. Purif. Technol. 2009, 70, 1–11. [Google Scholar] [CrossRef]
- Tharakan, A. Modelling of Physical and Chemical Processes in the Small Intestine. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2008. [Google Scholar]
- Ponchel, G.; Montisci, M.-J.; Dembri, A.; Durrer, C.; Duchêne, D. Mucoadhesion of colloidal particulate systems in the gastro-intestinal tract. Eur. J. Pharm. Biopharm. 1997, 44, 25–31. [Google Scholar] [CrossRef]
- Lee, Y.-A.L.; Zhang, S.; Lin, J.; Langer, R.; Traverso, G. A janus mucoadhesive and omniphobic device for gastrointestinal retention. Adv. Healthc. Mater. 2016, 5, 1141–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.; Zhang, L.; Zhang, G.; Meng, Q. Polymer membrane and cell models for drug discovery. Comb. Chem. High Throughput Scr. 2012, 15, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Lekang, O.-I. Aquaculture Engineering, 2nd ed.; Wiley-Blackwell: Chichester, West Sussex, UK, 2013; pp. 99–112. [Google Scholar]
- Mänttäri, M.; Pihlajamäki, A.; Kaipainen, E.; Nyström, M. Effect of temperature and membrane pre-treatment by pressure on the filtration properties of nanofiltration membranes. Desalination 2002, 145, 81–86. [Google Scholar] [CrossRef]
- Bargeman, G.; Westerink, J.B.; Guerra-Miguez, O.; Wessling, M. The effect of NaCl and glucose concentration on retentions for nanofiltration membranes processing concentrated solutions. Sep. Purif. Technol. 2014, 134, 46–57. [Google Scholar] [CrossRef]
- Luo, J.; Guo, S.; Wu, Y.; Wan, Y. Separation of sucrose and reducing sugar in cane molasses by nanofiltration. Food Bioprocess Tech. 2018, 11, 913–925. [Google Scholar] [CrossRef]
- Englyst, K.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar]
- Fisher, R.; Malmud, L.; Bandini, P.; Rock, E. Gastric emptying of a physiologic mixed solid-liquid meal. Clin. Nucl. Med. 1982, 7, 215–221. [Google Scholar] [CrossRef]
- Hellstrom, P.; Gryback, P.; Jacobsson, H. The physiology of gastric emptying. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 397–407. [Google Scholar] [CrossRef]
- Ferrua, M.J.; Singh, R.P. Human gastric simulator. In The Impact of Food Bioactives on Health. In Vitro and Ex Vivo Models, 1st ed.; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Heidelberg, Germany, 2015; pp. 61–71. [Google Scholar]
- Helander, H.; Fandriks, L. Surface area of the digestive tract–revisited. Scand. J. Gastroentero. 2014, 49, 681–689. [Google Scholar] [CrossRef]
- Normén, L.; Laerke, H.N.; Jensen, B.B.; Langkilde, A.M.; Anderson, H. Small-bowel absorption of D-tagatose and related effects on carbohydrate digestibility: An ileostomy study. Am. J. Clin. Nutr. 2001, 73, 105–110. [Google Scholar] [CrossRef]
- Kong, F.; Singh, R.P. A human gastric simulator (HGS) to study food digestion in human stomach. J. Food Sci. 2010, 75, E627–E635. [Google Scholar] [CrossRef]
- Schulze, K. Imaging and modelling of digestion in the stomach and the duodenum. Neurogastroent. Motil. 2006, 18, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Fagerholm, U.; Lennernas, H. Experimental estimation of the effective unstirred water layer thickness in the human jejunum, and its importance in oral-drug absorption. Eur. J. Pharm. Sci. 1995, 3, 247–253. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assuncao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]








| Energy Content (kcal) | 314 |
|---|---|
| Protein | 0.2 |
| Total fat | 0.1 |
| Total carbohydrate | 82.6 |
| Sodium | 0.0076 |
| Feed Flow/Dialysate Flow Ratio | Starch Digested (%) | Membrane Efficiency (%) | Expected Overall Efficiency (%) |
|---|---|---|---|
| 250/400 | 14.6 | 23.6 | 10.2 |
| 400/400 | 14.3 | 74.4 | 31.5 |
| 400/250 | 24.5 | 91.5 | 66.4 |
| Parameter | Human Small Intestine | Feed Flow/Dialysate Flow Ratio | ||
|---|---|---|---|---|
| 250/400 | 400/400 | 400/250 | ||
| Reducing sugars transfer, % | 96.4 1 | 24.1 | 24.1 | 24.1 |
| , mg/mL | 2.2 | 2.2 | 2.2 | 2.2 |
| , mg/mL | 0.079 | 0.50 | 0.62 | 0.37 |
| , mg/mL | 0 | 0.05 2 | 0.05 2 | 0.05 2 |
| , g | - | 3.41 | 3.17 | 3.66 |
| VF, L | 0.5 3 | 2 | 2 | 2 |
| Nrs, g | 1.06 | 1.06 | 1.06 | 1.06 |
| AT, m2 | 30 4 | 1.7 | 1.7 | 1.7 |
| ΔP, mmHg | - | −7.25 5 | 19.66 5 | 59.73 5 |
| , mg/mL | - | 1.2 | 1.1 | 1.3 |
| Feed flow, mL/min | 3 6 | 250 | 400 | 400 |
| Time, min | 180 7 | 30 | 64 | 30 |
| RMT | - | 2.3 | 5.5 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, C.; González, D.; Zúñiga, R.N.; Estay, H.; Troncoso, E. Simulation of Human Small Intestinal Digestion of Starch Using an In Vitro System Based on a Dialysis Membrane Process. Foods 2020, 9, 913. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9070913
González C, González D, Zúñiga RN, Estay H, Troncoso E. Simulation of Human Small Intestinal Digestion of Starch Using an In Vitro System Based on a Dialysis Membrane Process. Foods. 2020; 9(7):913. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9070913
Chicago/Turabian StyleGonzález, Carol, Daniela González, Rommy N. Zúñiga, Humberto Estay, and Elizabeth Troncoso. 2020. "Simulation of Human Small Intestinal Digestion of Starch Using an In Vitro System Based on a Dialysis Membrane Process" Foods 9, no. 7: 913. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9070913