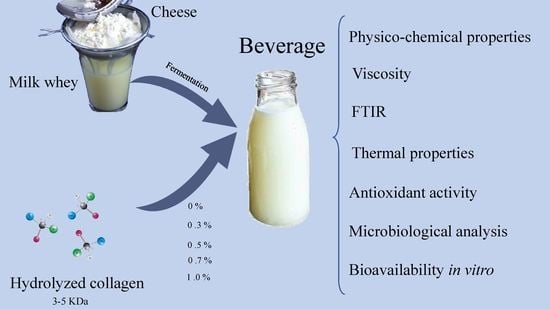
Characterization of Whey-Based Fermented Beverages Supplemented with Hydrolyzed Collagen: Antioxidant Activity and Bioavailability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Milk Whey Characterization
2.2. Hydrolyzed Collagen
2.3. Preparation of Beverage
2.4. Protein Determination
2.5. Fat Determination
2.6. pH Value
2.7. Lactose Determination
2.8. Ash Determination
2.9. Hydroxyproline Content
2.10. Viscosity Analysis
2.11. Fourier Transform-Infrared Spectroscopy
2.12. Differential Scanning Calorimetry
2.13. Antioxidant Activity
2.14. Microbiological Analysis
2.15. In Vitro Bioavailability of Hydrolyzed Collagen in Beverages
2.16. Statistical Analysis
3. Results and Discussion
3.1. Acid Milk Whey Chemical Characterization
3.2. Physicochemical Characterization of The Functional Beverage
3.3. Viscosity Determination of the Whey Beverage
3.4. Fourier Transform-Infrared Spectroscopy
3.5. Thermal Properties of Whey Beverage
3.6. Antioxidant Activity by ABTS and DPPH Radical Inhibition
3.7. Microbiological Analysis
3.8. In Vitro Bioavailability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| ANOVA | analysis of variance |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FTIR | Fourier transform-infrared spectroscopy |
| HC | hydrolyzed collagen |
| LAB- | lactic acid bacteria |
References
- Bigliardi, B.; Galati, F. Innovation trends in the food industry: The case of functional foods. Trends Food Sci. Technol. 2013, 31, 118–129. [Google Scholar] [CrossRef]
- Raman, M.; Ambalam, P.; Doble, M. 9-Probiotics, Prebiotics, and Fibers in Nutritive and Functional Beverages. In Nutrients in Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 315–367. [Google Scholar] [CrossRef]
- Hasler, C.M. Functional Foods: Benefits, Concerns and Challenges—A Position Paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef] [Green Version]
- Ofori, J.A.; Peggy, Y.-H. Novel Technologies for the Production of Functional Foods. In Bio-Nanotechnology: A Revolution in Food, Biomedical and Health Sciences; Shahidi, F., Bagchi, D., Bagchi, M., Moriyama, H., Shahidi, F., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 141–162. [Google Scholar]
- Özer, B.H.; Kirmaci, H.A. Functional milks and dairy beverages. Int. J. Dairy Technol. 2010, 63, 1–15. [Google Scholar] [CrossRef]
- Turkmen, N.; Akal, C.; Ozer, B. Probiotic dairy-based beverages: A review. J. Funct. Foods 2019, 53, 62–75. [Google Scholar] [CrossRef]
- Kareb, O.; Aïder, M. Whey and Its Derivatives for Probiotics, Prebiotics, Synbiotics, and Functional Foods: A Critical Review. Probiotics Antimicrob. Proteins 2019, 11, 348–369. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Song, M.; Hwang, S. Optimizing bioconversion of deproteinated cheese whey to mycelia of Ganoderma lucidum. Process. Biochem. 2003, 38, 1685–1693. [Google Scholar] [CrossRef]
- Ásványi, B.; Reichart, O.; Szigeti, J.; Varga, L. Screening and selection of Kluyveromyces strains for use in batch production of single-cell protein from cheese whey. Milchwissenschaft 2006, 61, 378–381. [Google Scholar]
- Alvarado Carrasco, C.; Guerra, M. Lactosuero como fuente de péptidos bioactivos. Anales Venez. Nutr. 2010, 23, 45–50. [Google Scholar]
- Chatterjee, G.; De Neve, J.; Dutta, A.; Das, S. Formulation and statistical evaluation of a ready-to-drink whey based orange beverage and its storage stability. Rev. Mex. Ing. Quím. 2015, 14, 253–264. [Google Scholar]
- Ramírez-Navas, J.S. Aprovechamiento Industrial de Lactosuero Mediante Procesos Fermentativos. Publ. Investig. 2012, 6, 69–83. [Google Scholar] [CrossRef] [Green Version]
- Vivas, Y.A.; Morales, A.J.; Otálvaro, Á.M. Aprovechamiento de lactosuero para el desarrollo de una bebida refrescante con antioxidantes naturales. Aliment. Hoy 2017, 24, 185–199. [Google Scholar]
- Pescuma, M.; Hebert, E.; Mozzi, F.; De Valdez, G.F. Whey fermentation by thermophilic lactic acid bacteria: Evolution of carbohydrates and protein content. Food Microbiol. 2008, 25, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Skov, K.; Oxfeldt, M.; Thøgersen, R.; Hansen, M.G.; Bertram, H.C. Enzymatic Hydrolysis of a Collagen Hydrolysate Enhances Postprandial Absorption Rate-A Randomized Controlled Trial. Nutrients 2019, 11, 1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [Green Version]
- Aguirre-Cruz, G.; León-López, A.; Cruz-Gómez, V.; Alvarado, R.J.; Aguirre-Álvarez, G. Collagen Hydrolysates for Skin Protection: Oral Administration and Topical Formulation. Antioxidants 2020, 9, 181. [Google Scholar] [CrossRef] [Green Version]
- Najafian, L.; Babji, A. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef]
- Bradford, N. A rapid and sensitive method for the quantitation microgram quantities of a protein isolated from red cell membranes. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. pH Determination, 18th ed.; AOAC: Gaithersburg, MD, USA, 1997. [Google Scholar]
- AOAC. Official Methods of Analysis. Fat Determination, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Trevelyan, W.E.; Harrison, J.S. Studies on yeast metabolism. Fractionation and microdetermination of cell carbohydrates. Biochem. J. 1952, 50, 298–303. [Google Scholar] [CrossRef]
- León-López, A.; Fuentes-Jiménez, L.; Fuentes, A.D.H.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Hydrolysed Collagen from Sheepskins as a Source of Functional Peptides with Antioxidant Activity. Int. J. Mol. Sci. 2019, 20, 3931. [Google Scholar] [CrossRef] [Green Version]
- Tirado-Armesto, D.F. Elaboración de una bebida láctea a base de lactosuero fermentado usando Streptococcus salivarius ssp., Thermophilus y Lactobacillus casei ssp. casei. Cienc. Tecnol. Aliment. 2015, 13, 13–19. [Google Scholar]
- López-Legarda, X.; Taramuel-Gallardo, A.; Arboleda-Echavarría, C.; Segura-Sánchez, F.; Restrepo-Betancur, L.F. Comparación de métodos que utilizan ácido sulfúrico para la determinación de azúcares totales. Rev. Cuba. Quími. 2017, 29, 180–198. [Google Scholar]
- AOAC. Official Methods of Analysis. Ashes Determination, 16th ed.; AOAC: Washington, DC, USA, 1995. [Google Scholar]
- AOAC. Official Methods of Analysis. Amino Acids, 17th ed.; AOAC: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Boil. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bilek, S.E.; Bayram, S.K. Fruit juice drink production containing hydrolyzed collagen. J. Funct. Foods 2015, 14, 562–569. [Google Scholar] [CrossRef]
- Poveda, E. Suero lácteo, generalidades y potencial uso como fuente de calcio de alta biodisponibilidad. Rev. Chil. Nutr. 2013, 40, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Skryplonek, K. The use of acid whey for the production of yogurt-type fermented beverages. Mljek. Čas. Unapr. Proizv. Prerade Mlijek. 2018, 68, 139–149. [Google Scholar]
- Atallah, A.A.; Gemiel, D.G. Preparation of New Carbonated Beverages Based on Hydrolyzed Whey by Fruit and Some Herbs. Am. J. Food Sci. Technol. 2020, 8, 49–55. [Google Scholar]
- Huertas, R.A.P. Lactosuero: Importancia en la industria de alimentos. Rev. Fac. Nac. Agron. Medellín 2009, 62, 4967–4982. [Google Scholar]
- Jelen, P. Industrial whey processing technology: An overview. J. Agric. Food Chem. 1979, 27, 658–661. [Google Scholar] [CrossRef]
- Smith, T.; Foegeding, E.; Drake, M. Flavor and Functional Characteristics of Whey Protein Isolates from Different Whey Sources. J. Food Sci. 2016, 81, C849–C857. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Carvalho, M.; Rivas, J.; Carvalho, F. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Macwan, S.R.; Dabhi, B.K.; Parmar, S.; Aparnathi, K. Whey and its Utilization. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 134–155. [Google Scholar] [CrossRef]
- Mann, B.; Kumari, A.; Kumar, R.; Sharma, R.; Prajapati, K.; Mahboob, S.; Athira, S. Antioxidant activity of whey protein hydrolysates in milk beverage system. J. Food Sci. Technol. 2014, 52, 3235–3241. [Google Scholar] [CrossRef] [PubMed]
- Skryplonek, K.; Dmytrów, I.; Mituniewicz-Małek, A. Probiotic fermented beverages based on acid whey. J. Dairy Sci. 2019, 102, 7773–7780. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, P.M.; Teixeira, J.A.; Domingues, L. Fermentation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorisation of cheese whey. Biotechnol. Adv. 2010, 28, 375–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hailu, M.; Tola, A.; Teshome, G.; Agza, B. Development of Beverages from Traditional Whey and Natural Fruit Juices. Food Sci. Nutr. Complet. Res. 2019, 85–93. Available online: http://publication.eiar.gov.et:8080/xmlui/bitstream/handle/123456789/3288/Food%20Science%20Proceedings-pdg.pdf?sequence=1&isAllowed=y#page=89 (accessed on 10 August 2020).
- De Lima, A.V.S.C.; Nicolau, E.S.; Rezende, C.S.M.E.; Torres, M.C.L.; Novais, L.G.; Soares, N.R. Characterization and sensory preference of fermented dairy beverages prepared with different concentrations of whey and araticum pulp. Semin. Ciênc. Agrár. 2016, 37, 4011. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.T.S.E.; Spadoti, L.M.; Zacarchenco, P.B.; Trento, F.K.H.S.E.; Alves, A.S. Probiotic Functional Carbonated Whey Beverages: Development and Quality Evaluation. Beverages 2018, 4, 49. [Google Scholar] [CrossRef] [Green Version]
- Saha, P.; Ray, P.; Ghatak, P.; Bag, S.; Hazra, T. Physico-chemical quality and storage stability of fermented Chhana whey beverages. Indian J. Dairy Sci. 2017, 70, 398–403. [Google Scholar]
- Akpinar, A.; Torunoglu, F.A.; Yerlikaya, O.; Kinik, O.; Akbulut, N.; Uysal, H.R. Fermented probiotic beverages produced with reconstituted whey and cow milk. Agro FOOD Ind. Hi Tech. 2015, 26, 4. [Google Scholar]
- Schrieber, R.; Gareis, H. Gelatine Handbook: Theory and Industrial Practice; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Gerhardt, Â.; Monteiro, B.W.; Gennari, A.; Lehn, D.N.; de Souza, C.F.V. CARACTERÍSTICAS FÍSICO-QUÍMICAS E SENSORIAIS DE BEBIDAS LÁCTEAS FERMENTADAS UTILIZANDO SORO DE RICOTA E COLÁGENO HIDROLISADO Physicochemical and sensory characteristics of fermented dairy drink using ricotta cheese whey and hydrolyzed collagen. Rev. Inst. Laticínios Cândido Tostes 2013, 68, 41–50. [Google Scholar] [CrossRef]
- Rigoto, J.D.M.; Ribeiro, T.H.; Stevanato, N.; Sampaio, A.R.; Ruiz, S.; Bolanho, B.C. Effect of açaí pulp, cheese whey, and hydrolysate collagen on the characteristics of dairy beverages containing probiotic bacteria. J. Food Process. Eng. 2019, 42, e12953. [Google Scholar] [CrossRef] [Green Version]
- López, P.I.G.; Zambrano Ángela, M.Z.; Rosado, C.F.R.; Peña, A.M. Evaluación de una bebida láctea fermentada novel a base de lactosuero y harina de camote. La Téc. 2018, 19, 47–60. [Google Scholar]
- Chairunnisa, H.; Balia, R.L.; Wulandari, E. Chemical and Microbiological Characteristics of Fermented Cheese Whey Beverages with Soymilk Powder Addition. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 334, p. 012043. [Google Scholar]
- Imbachí-Narváez, P.C.; Sepúlveda-Valencia, J.U.; Rodriguez-Sandoval, E. Effect of modified cassava starch on the rheological and quality properties of a dairy beverage prepared with sweet whey. Food Sci. Technol. 2019, 39, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Fanelli, S.; Zimmermann, A.; Tótoli, E.G.; Salgado, H.R.N. FTIR Spectrophotometry as a Green Tool for Quantitative Analysis of Drugs: Practical Application to Amoxicillin. J. Chem. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Andrade, J.; Pereira, C.G.; Junior, J.C.D.A.; Viana, C.C.R.; Neves, L.N.D.O.; Da Silva, P.H.F.; Bell, M.J.V.; Anjos, V.D.C.D. FTIR-ATR determination of protein content to evaluate whey protein concentrate adulteration. LWT 2019, 99, 166–172. [Google Scholar] [CrossRef]
- Ferraro, V.; Madureira, A.R.; Sarmento, B.; Gomes, A.M.; Pintado, M.E. Study of the interactions between rosmarinic acid and bovine milk whey protein α-Lactalbumin, β-Lactoglobulin and Lactoferrin. Food Res. Int. 2015, 77, 450–459. [Google Scholar] [CrossRef]
- Salari, M.; Khiabani, M.S.; Mokarram, R.R.; Ghanbarzadeh, B.; Kafil, H.S.; Khiabani, M.S. Preparation and characterization of cellulose nanocrystals from bacterial cellulose produced in sugar beet molasses and cheese whey media. Int. J. Boil. Macromol. 2019, 122, 280–288. [Google Scholar] [CrossRef]
- Wang, T.; Tan, S.-Y.; Mutilangi, W.; Plans, M.; Rodriguez-Saona, L. Application of infrared portable sensor technology for predicting perceived astringency of acidic whey protein beverages. J. Dairy Sci. 2016, 99, 9461–9470. [Google Scholar] [CrossRef] [Green Version]
- Arruda, H.S.; Silva, E.K.; Pereira, G.A.; Meireles, M.A.A.; Pastore, G.M. Inulin thermal stability in prebiotic carbohydrate-enriched araticum whey beverage. LWT 2020, 128, 109418. [Google Scholar] [CrossRef]
- Fitzsimons, S.M.; Mulvihill, D.M.; Morris, E.R. Denaturation and aggregation processes in thermal gelation of whey proteins resolved by differential scanning calorimetry. Food Hydrocoll. 2007, 21, 638–644. [Google Scholar] [CrossRef]
- Boostani, S.; Aminlari, M.; Moosavi-Nasab, M.; Niakosari, M.; Mesbahi, G. Fabrication and characterisation of soy protein isolate-grafted dextran biopolymer: A novel ingredient in spray-dried soy beverage formulation. Int. J. Boil. Macromol. 2017, 102, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Agyare, K.K.; Damodaran, S. pH-Stability and Thermal Properties of Microbial Transglutaminase-Treated Whey Protein Isolate. J. Agric. Food Chem. 2010, 58, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, Z.; Li, T.; Sarker, S.-K.; Chowdhury, S.; Jiang, Z.; Mu, Z. Effects of pH Values on Physicochemical Properties and Antioxidant Potential of Whey Protein Isolate-safflower Yellow Complexes. Food Sci. Technol. Res. 2018, 24, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Joyce, A.M.; Kelly, A.L.; O’Mahony, J.A.; Brodkorb, A. Separation of the effects of denaturation and aggregation on whey-casein protein interactions during the manufacture of a model infant formula. Dairy Sci. Technol. 2016, 96, 787–806. [Google Scholar] [CrossRef] [Green Version]
- Vieira, A.H.; Balthazar, C.F.; Guimaraes, J.T.; Rocha, R.S.; Pagani, M.M.; Esmerino, E.A.; Silva, M.C.; Raices, R.S.; Tonon, R.V.; Cabral, L.M.; et al. Advantages of microfiltration processing of goat whey orange juice beverage. Food Res. Int. 2020, 132, 109060. [Google Scholar] [CrossRef]
- Chen, H.-M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative Properties of Histidine-Containing Peptides Designed from Peptide Fragments Found in the Digests of a Soybean Protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino Acid Composition and Antioxidant Properties of Pea Seed (Pisum sativum L.) Enzymatic Protein Hydrolysate Fractions. J. Agric. Food Chem. 2010, 58, 4712–4718. [Google Scholar] [CrossRef]
- Arranz, E.; Corrochano, A.; Shanahan, C.; Villalva, M.; Jaime, L.; Santoyo, S.; Callanan, M.; Murphy, E.; Giblin, L. Antioxidant activity and characterization of whey protein-based beverages: Effect of shelf life and gastrointestinal transit on bioactivity. Innov. Food Sci. Emerg. Technol. 2019, 57, 102209. [Google Scholar] [CrossRef]
- Arsić, S.; Bulatović, M.; Zarić, D.; Kokeza, G.; Subić, J.; Rakin, M. Functional fermented whey carrot beverage-qualitative, nutritive and techno-economic analysis. Rom. Biotechnol. Lett. 2018, 23, 13496–13504. [Google Scholar]
- Sady, M.; Jaworska, G.; Grega, T.; Bernas, E.; Domagala, J. Application of acid whey in orange drink production. Food Technol. Biotechnol. 2013, 51, 266. [Google Scholar]
- Guimarães, J.T.; Silva, E.K.; Ranadheera, C.S.; Moraes, J.; Raices, R.S.; Silva, M.C.; Ferreira, M.S.; Freitas, M.Q.; Meireles, M.A.A.; Cruz, A.G. Effect of high-intensity ultrasound on the nutritional profile and volatile compounds of a prebiotic soursop whey beverage. Ultrason. Sonochem. 2019, 55, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.V.S.; Cappato, L.P.; Silva, R.; Rocha, R.S.; Guimarães, J.T.; Balthazar, C.F.; Esmerino, E.A.; Freitas, M.Q.; Rodrigues, F.N.; Granato, D.; et al. Ohmic heating for processing of whey-raspberry flavored beverage. Food Chem. 2019, 297, 125018. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.; Montero, M. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Vij, S.; Singh, B.P. Antioxidative and antimicrobial activity of whey based fermented soy beverage with curcumin supplementation. Indian J. Dairy Sci. 2016, 69, 171–177. [Google Scholar]
- Valadao, N.K.; De Andrade, I.M.G. Development of a Ricotta Cheese Whey-based Sports Drink. Adv. Dairy Res. 2016, 4, 156. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J.D.C.G.; Mendonça, A.C.; Viana, K.W.C.; Maia, M.D.P.; De Carvalho, A.; Minim, V.P.R.; Stringheta, P.C. Beverages formulated with whey protein and added lutein. Ciênc. Rural 2017, 47. [Google Scholar] [CrossRef] [Green Version]
- Benjakul, S.; Chantakun, K.; Karnjanapratum, S. Impact of retort process on characteristics and bioactivities of herbal soup based on hydrolyzed collagen from seabass skin. J. Food Sci. Technol. 2018, 55, 3779–3791. [Google Scholar] [CrossRef]
- Sousa, S.C.; Fragoso, S.P.; Penna, C.R.; Arcanjo, N.M.; Silva, F.A.; Ferreira, V.C.; Barreto, M.D.; Araújo, B.S. Quality parameters of frankfurter-type sausages with partial replacement of fat by hydrolyzed collagen. LWT Food Sci. Technol. 2017, 76, 320–325. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef]
- Lutz, M. Biodisponibilidad de compuestos bioactivos en alimentos. Perspect. Nutr. Hum. 2013, 15, 217–226. [Google Scholar]
- Hajirostamloo, B. Bioactive component in milk and dairy product. World Acad. Sci. Eng. Technol. 2010, 72, 162–166. [Google Scholar]
- Feng, M.; Betti, M. Transepithelial transport efficiency of bovine collagen hydrolysates in a human Caco-2 cell line model. Food Chem. 2017, 224, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Sontakke, S.B.; Jung, J.-H.; Piao, Z.; Chung, H.J. Orally Available Collagen Tripeptide: Enzymatic Stability, Intestinal Permeability, and Absorption of Gly-Pro-Hyp and Pro-Hyp. J. Agric. Food Chem. 2016, 64, 7127–7133. [Google Scholar] [CrossRef] [PubMed]
- Clemente, A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci. Technol. 2000, 11, 254–262. [Google Scholar] [CrossRef]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. Characterization of protein hydrolysates from blue whiting (Micromesistius poutassou) and their application in beverage fortification. Food Chem. 2018, 245, 698–706. [Google Scholar] [CrossRef]
- Ganapathy, V.; Gupta, N.; Martindale, R.G. Protein Digestion and Absorption. In Physiolog of the Gastrointestinal Tract; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1667–1692. [Google Scholar]
- Moskowitz, R.W. Role of collagen hydrolysate in bone and joint disease. Semin. Arthritis Rheum. 2000, 30, 87–99. [Google Scholar] [CrossRef]




| Hydrolyzed Collagen Concentration (%) | pH | Ash (%) | Fat (g/L) | Lactose (g/L) | Protein (g/L) |
|---|---|---|---|---|---|
| 0 | 5.10 ± 0.03 b | 0.82 ± 0.11 c | 0.20 ± 0.10 a | 27.09 ± 10.63 a | 9.13 ± 0.09 c |
| 0.3 | 7.07 ± 0.01 a | 0.83 ± 0.11 bc | 0.23 ± 0.06 a | 18.65 ± 8.60 a | 9.35 ± 0.10 b |
| 0.5 | 7.03 ± 0.03 a | 1.14 ± 0.02 ab | 0.27 ± 0.06 a | 20.10 ± 3.34 a | 9.45 ± 0.07 b |
| 0.7 | 7.36 ± 0.03 a | 1.03 ± 0.01 a | 0.27 ± 0.06 a | 15.12 ± 3.62 a | 9.48 ± 0.11 b |
| 1.0 | 7.39 ± 0.03 a | 1.19 ± 0.06 a | 0.23 ± 0.06 a | 28.26 ± 6.31 a | 9.75 ± 0.20 a |
| Hydrolyzed Collagen Concentration (%) | ∆H (J/g) | Tm (°C) |
|---|---|---|
| 0 | 94.81 ± 6.01 a | 163.504 ± 5.16 a |
| 0.3 | 12.77 ± 4.23 b | 158.88 ± 5.84 a |
| 0.5 | 8.80 ± 6.07 b | 147.03 ± 4.43 a |
| 0.7 | 11.53 ± 2.26 b | 165.612 ± 2.75 a |
| 1.0 | 14.818 ± 3.37 b | 161.4 ± 5.90 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-López, A.; Pérez-Marroquín, X.A.; Campos-Lozada, G.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Characterization of Whey-Based Fermented Beverages Supplemented with Hydrolyzed Collagen: Antioxidant Activity and Bioavailability. Foods 2020, 9, 1106. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9081106
León-López A, Pérez-Marroquín XA, Campos-Lozada G, Campos-Montiel RG, Aguirre-Álvarez G. Characterization of Whey-Based Fermented Beverages Supplemented with Hydrolyzed Collagen: Antioxidant Activity and Bioavailability. Foods. 2020; 9(8):1106. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9081106
Chicago/Turabian StyleLeón-López, Arely, Xóchitl Alejandra Pérez-Marroquín, Gieraldin Campos-Lozada, Rafael G. Campos-Montiel, and Gabriel Aguirre-Álvarez. 2020. "Characterization of Whey-Based Fermented Beverages Supplemented with Hydrolyzed Collagen: Antioxidant Activity and Bioavailability" Foods 9, no. 8: 1106. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9081106