Phytotoxicity of Essential Oils: Opportunities and Constraints for the Development of Biopesticides. A Review
Abstract
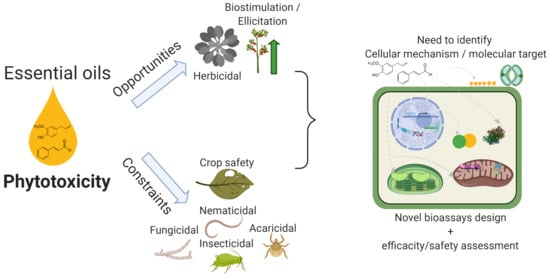
:1. Introduction
2. Essential Oils’ Cellular and Physiological Impacts
2.1. Essential Oils’ Translocation
2.2. Water Status Alteration
2.3. Membrane Properties and Interactions
2.4. Reactive Oxygen and Nitrogen Species Induction
2.5. Photosynthesis Inhibition
2.6. Mitochondrial Respiration Inhibition
2.7. Microtubule Disruption and Genotoxicity
2.8. Enzymatic Inhibition and Regulation
2.9. Phytohormones and Priming of Plant Defence
3. Mechanism of Detoxification
4. Discussion and Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PPP | plant protection product |
| EO(s) | essential oil(s) |
| VOCs | volatile organic compounds |
| EOC | essential oil constituents |
| IPM | integrated pest management |
| ATP | adenosine triphosphate |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| H2O2 | hydrogen peroxide |
| MDA | Malondialdehyde |
| LOX | lipoxygenase |
| EL | electrolyte leakage |
| PS | photosystem |
| GAs | gibberellins |
| TAT | tyrosine aminotransferase |
| PR | pathogenesis related |
| SAR | systemic acquired resistance |
| ISR | induced systemic resistance |
| SA | salicylic acid |
| JA | jasmonic acid |
| GLV | green leaf volatiles |
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.S.; Wang, L. The phenylpropanoid pathway and plant defence-a genomics perspective. Mol. Plant. Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, C.A.; Pendleton, S.J.; Crandall, P.G.; Ricke, S.C. Potential of plant essential oils and their components in animal agriculture—In vitro studies on antibacterial mode of action. Front. Vet. Sci. 2015, 2, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavanagh, H. Antifungal activity of the volatile phase of essential oils: A brief review. Nat. Prod. Commun. 2007, 2, 1297–1302. [Google Scholar] [CrossRef] [Green Version]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular targets for components of essential oils in the insect nervous system—A review. Molecules 2018, 23, 34. [Google Scholar] [CrossRef] [Green Version]
- Peixoto, M.G.; Costa-Junior, L.M.; Blank, A.F.; Lima, A.D.S.; Menezes, T.S.A.; Santos, D.D.A.; Alves, P.B.; Cavalcanti, S.C.D.H.; Bacci, L.; Arrigoni-Blank, M.D.F. Acaricidal activity of essential oils from Lippia alba genotypes and its major components carvone, limonene, and citral against Rhipicephalus microplus. Vet. Parasitol. 2017, 210, 118–122. [Google Scholar] [CrossRef]
- Andrés, M.F.; González-Coloma, A.; Sanz, J.; Burillo, J.; Sainz, P. Nematicidal activity of essential oils: A review. Phytochem. Rev. 2012, 11, 371–390. [Google Scholar] [CrossRef] [Green Version]
- Tworkoski, T. Herbicide effects of essential oils. Weed Sci. 2002, 50, 425–431. [Google Scholar] [CrossRef]
- Koul, O.; Walia, S.; Dhaliwal, G. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Isman, M.B. Plant essential oils for pest and disease management. Crop. Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Phytotoxic effects of commercial essential oils on selected vegetable crops: Cucumber and tomato. Sustain. Chem. Pharm. 2020, 15, 100209. [Google Scholar] [CrossRef]
- Isman, M.B.; Zheljazkov, V.; Cantrell, C.L. Pesticides based on plant essential oils: Phytochemical and practical considerations. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2016; Volume 1228, pp. 13–26. [Google Scholar]
- Domingues, P.M.; Santos, L. Essential oil of pennyroyal (Mentha pulegium): Composition and applications as alternatives to pesticides—New tendencies. Ind. Crop. Prod. 2019, 139, 111534. [Google Scholar] [CrossRef]
- Rolli, E.; Marieschi, M.; Maietti, S.; Sacchetti, G.; Bruni, R. Comparative phytotoxicity of 25 essential oils on pre- and post-emergence development of Solanum lycopersicum L.: A multivariate approach. Ind. Crop. Prod. 2014, 60, 280–290. [Google Scholar] [CrossRef]
- Topuz, E.; Madanlar, N.; Erler, F. Chemical composition, toxic and development- and reproduction-inhibiting effects of some essential oils against Tetranychus urticae Koch (Acarina: Tetranychidae) as fumigants. J. Plant. Dis. Prot. 2018, 125, 377–387. [Google Scholar] [CrossRef]
- Yan, Z.Q.; Wang, D.-D.; Ding, L.; Cui, H.Y.; Jin, H.; Yang, X.Y.; Yang, J.S.; Qin, B. Mechanism of artemisinin phytotoxicity action: Induction of reactive oxygen species and cell death in lettuce seedlings. Plant. Physiol. Biochem. 2015, 88, 53–59. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, A.K. Prospective of essential oils of the genus Mentha as biopesticides: A review. Front. Plant. Sci. 2018, 9, 1295. [Google Scholar] [CrossRef]
- Grana, E.; Sánchez-Moreiras, A.M.; Reigosa, M.J. Mode of action of monoterpenes in plant-plant interactions. Curr. Bioact. Compd. 2012, 8, 80–89. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Jaini, R.; Morgan, J.A.; Dudareva, N. Rethinking how volatiles are released from plant cells. Trends Plant. Sci. 2015, 20, 545–550. [Google Scholar] [CrossRef] [Green Version]
- Maffei, M.E. Sites of synthesis, biochemistry and functional role of plant volatiles. S. Afr. J. Bot. 2010, 76, 612–631. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.; Fu, X.; Watanabe, N.; Su, X.; Yang, Z. Recent advances in the emission and functions of plant vegetative volatiles. Molecules 2016, 21, 124. [Google Scholar] [CrossRef]
- Sugimoto, K.; Matsui, K.; Takabayashi, J.; Blande, J.D.; Glinwood, R. Uptake and Conversion of Volatile Compounds in Plant–Plant Communication; Springer: Cham, Switzerland, 2016; pp. 305–316. [Google Scholar]
- Adebesin, F.; Widhalm, J.R.; Boachon, B.; Lefèvre, F.; Pierman, B.; Lynch, J.H.; Alam, I.; Junqueira, B.; Benke, R.; Ray, S.; et al. Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter. Science 2017, 356, 1386–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.R.; Coats, J.R. Movement of thymol in citrus plants. In Roles of Natural Products for Biorational Pesticides in Agriculture; American Chemical Society: Washington, DC, USA, 2018; Volume 1294, pp. 23–32. [Google Scholar]
- Schulz, M.; Kussmann, P.; Knop, M.; Kriegs, B.; Gresens, F.; Eichert, T.; Ulbrich, A.; Marx, F.; Fabricius, H.; Goldbach, H.; et al. Allelopathic monoterpenes interfere with Arabidopsis thaliana cuticular waxes and enhance transpiration. Plant. Signal. Behav. 2007, 2, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriegs, B.; Jansen, M.; Hahn, K.; Peisker, H.; Šamajová, O.; Beck, M.; Braun, S.; Ulbrich, A.; Baluška, F.; Schulz, M. Plant Signaling & Behavior Cyclic monoterpene mediated modulations of Arabidopsis thaliana phenotype Effects on the cytoskeleton and on the expression of selected genes. Plant Signal. Behav. 2010, 5, 832–838. [Google Scholar]
- Bainard, L.D.; Isman, M.B.; Upadhyaya, M.K. Phytotoxicity of clove oil and its primary constituent eugenol and the role of leaf epicuticular wax in the susceptibility to these essential oils. Weed Sci. 2006, 54, 833–837. [Google Scholar] [CrossRef]
- Grana, E.; Díaz-Tielas, C.; López-González, D.; Martínez-Peñalver, A.; Reigosa, M.J.; Sánchez-Moreiras, A.M. The plant secondary metabolite citral alters water status and prevents seed formation in Arabidopsis thaliana. Plant. Biol. 2016, 18, 423–432. [Google Scholar] [CrossRef]
- Araniti, F.; Sánchez-Moreiras, A.M.; Graña, E.; Reigosa, M.J.; Abenavoli, M.R. Terpenoid trans-caryophyllene inhibits weed germination and induces plant water status alteration and oxidative damage in adult Arabidopsis. Plant Biol. 2017, 19, 79–89. [Google Scholar] [CrossRef]
- Griffin, S.; Markham, J.L.; Dennis, G.; Grant Wyllie, S. Using atomic force microscopy to view the effects of terpenoids on the stability and packing of phosphatidylcholine supported lipid bilayers. In Proceedings of the 31st International Symposium on Essential Oils, Hamburg, Germany, 10–13 September 2000. [Google Scholar]
- Maffei, M.E.; Camusso, W.; Sacco, S. Effect of Mentha x piperita essential oil and monoterpenes on cucumber root membrane potential. Phytochemistry 2001, 58, 703–707. [Google Scholar] [CrossRef]
- Marcec, M.J.; Gilroy, S.; Poovaiah, B.; Tanaka, K. Mutual interplay of Ca2+ and ROS signaling in plant immune response. Plant. Sci. 2019, 283, 343–354. [Google Scholar] [CrossRef]
- Turina, A.V.; Nolan, M.; Zygadlo, J.; Perillo, M. Natural terpenes: Self-assembly and membrane partitioning. Biophys. Chem. 2006, 122, 101–113. [Google Scholar] [CrossRef]
- Witzke, S.; Duelund, L.; Kongsted, J.; Petersen, M.; Mouritsen, O.G.; Khandelia, H. Inclusion of terpenoid plant extracts in lipid bilayers investigated by molecular dynamics simulations. J. Phys. Chem. B 2010, 114, 15825–15831. [Google Scholar] [CrossRef]
- Lins, L.; Maso, S.D.; Foncoux, B.; Kamili, A.; Laurin, Y.; Genva, M.; Jijakli, M.H.; Fauconnier, M.L.; Fauconnier, M.L.; Deleu, M. Insights into the relationships between herbicide activities, molecular structure and membrane interaction of cinnamon and citronella essential oils components. Int. J. Mol. Sci. 2019, 20, 4007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zygadlo, J.A. Effect of monoterpenes on lipid oxidation in maize. Planta 2004, 219, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Zunino, M.P.; Zygadlo, J.A. Changes in the composition of phospholipid fatty acids and sterols of maize root in response to monoterpenes. J. Chem. Ecol. 2005, 31, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Gniazdowska, A.; Krasuska, U.; Andrzejczak, O.; Sołtys-Kalina, D. Allelopathic compounds as oxidative stress agents: Yes or no. In Signaling and Communication in Plants; Springer: Cham, Switzerland, 2015; pp. 155–176. [Google Scholar]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2000, 26, 2079–2094. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant. Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Sunohara, Y.; Baba, Y.; Matsuyama, S.; Fujimura, K.; Matsumoto, H. Screening and identification of phytotoxic volatile compounds in medicinal plants and characterizations of a selected compound, eucarvone. Protoplasma 2015, 252, 1047–1059. [Google Scholar] [CrossRef]
- Chowhan, N.; Singh, H.P.; Batish, D.R.; Kaur, S.; Ahuja, N.; Kohli, R.K. β-Pinene inhibited germination and early growth involves membrane peroxidation. Protoplasma 2013, 250, 691–700. [Google Scholar] [CrossRef]
- Chowhan, N.; Bali, A.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Reactive oxygen species generation and antioxidant defense system in hydroponically grown wheat (Triticum aestivum) upon β-pinene exposure: An early time course assessment. Acta Physiol. Plant. 2014, 36, 3137–3146. [Google Scholar] [CrossRef]
- Kaur, S.; Rana, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Citronellol disrupts membrane integrity by inducing free radical generation. Z. Naturforsch. Sect. C J. Biosci. 2011, 66, 260–266. [Google Scholar] [CrossRef]
- Dahiya, S.; Batish, D.R.; Singh, H.P. Pogostemon benghalensis essential oil inhibited the weed growth via causing oxidative damage. Braz. J. Bot. 2020, 43, 1–11. [Google Scholar] [CrossRef]
- Ricci, D.; Epifano, F.; Fraternale, D. The essential oil of Monarda didyma L. (Lamiaceae) exerts phytotoxic activity in vitro against various weed seed. Molecules 2017, 22, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Artemisia scoparia essential oil inhibited root growth involves reactive oxygen species (ROS)-mediated disruption of oxidative metabolism: In vivo ROS detection and alterations in antioxidant enzymes. Biochem. Syst. Ecol. 2012, 44, 390–399. [Google Scholar] [CrossRef]
- Lazarotto, D.C.; Pawlowski, Â.; Da Silva, E.R.; Schwambach, J.; Soares, G.L.G. Phytotoxic effects of Heterothalamus psiadioides (Asteraceae) essential oil on adventitious rooting. Acta Physiol. Plant. 2014, 36, 3163–3171. [Google Scholar] [CrossRef]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global plant stress signaling: Reactive oxygen species at the cross-road. Front. Plant. Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Arora, K.; Kohli, R.K. α-Pinene inhibits growth and induces oxidative stress in roots. Ann. Bot. 2006, 98, 1261–1269. [Google Scholar] [CrossRef] [Green Version]
- Pauly, G.; Douce, R.; Carde, J.P. Effects of β-pinene on spinach chloroplast photosynthesis. Z. Pflanzenphysiol. 1981, 104, 199–206. [Google Scholar] [CrossRef]
- Klingler, H.; Frosch, S.; Wagner, E. In vitro effects of monoterpenes on chloroplast membranes. Photosynth. Res. 1991, 28, 109–118. [Google Scholar] [CrossRef]
- Chowhan, N.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phytotoxic effects of β-pinene on early growth and associated biochemical changes in rice. Acta Physiol. Plant. 2011, 33, 2369–2376. [Google Scholar] [CrossRef]
- Poonpaiboonpipat, T.; Pangnakorn, U.; Suvunnamek, U.; Teerarak, M.; Charoenying, P.; Laosinwattana, C. Phytotoxic effects of essential oil from Cymbopogon citratus and its physiological mechanisms on barnyardgrass (Echinochloa crus-galli). Ind. Crop. Prod. 2013, 41, 403–407. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Chemical profiling, cytotoxicity and phytotoxicity of foliar volatiles of Hyptis suaveolens. Ecotoxicol. Environ. Saf. 2019, 171, 863–870. [Google Scholar] [CrossRef]
- Araniti, F.; Grana, E.; Krasuska, U.; Bogatek, R.; Reigosa, M.J.; Abenavoli, M.R.; Sánchez-Moreiras, A.M. Loss of gravitropism in farnesene-treated Arabidopsis is due to microtubule malformations related to hormonal and ROS unbalance. PLoS ONE 2016, 11, e0160202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouresmaeil, M.; Nojadeh, M.S.; Movafeghi, A.; Maggi, F. Exploring the bio-control efficacy of Artemisia fragrans essential oil on the perennial weed Convolvulus arvensis: Inhibitory effects on the photosynthetic machinery and induction of oxidative stress. Ind. Crop. Prod. 2020, 155, 112785. [Google Scholar] [CrossRef]
- Synowiec, A.; Możdżeń, K.; Skoczowski, A. Early physiological response of broccoli leaf to foliar application of clove oil and its main constituents. Ind. Crop. Prod. 2015, 74, 523–529. [Google Scholar] [CrossRef]
- Araniti, F.; Landi, M.; Lupini, A.; Sunseri, F.; Guidi, L.; Abenavoli, M.R. Origanum vulgare essential oils inhibit glutamate and aspartate metabolism altering the photorespiratory pathway in Arabidopsis thaliana seedlings. J. Plant. Physiol. 2018, 231, 297–309. [Google Scholar] [CrossRef]
- Muller, W.H.; Lorber, P.; Haley, B.; Johnson, K. Volatile growth inhibitors produced by Salvia leucophylla: Effect on oxygen uptake by mitochondrial suspensions. Bull. Torrey Bot. Club 1969, 96, 89. [Google Scholar] [CrossRef]
- Peñuelas, J.; Ribas-Carbo, M.; Giles, L. Effects of allelochemicals on plant respiration and oxygen isotope fractionation by the alternative oxidase. J. Chem. Ecol. 1996, 22, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Abrahim, D.; Braguini, W.L.; Kelmer-Bracht, A.M.; Ishii-Iwamoto, E.L. Effects of four monoterpenes on germination, primary root growth, and mitochondrial respiration of maize. J. Chem. Ecol. 2000, 26, 611–624. [Google Scholar] [CrossRef]
- Abrahim, D.; Francischini, A.C.; Pergo, E.M.; Kelmer-Bracht, A.M.; Ishii-Iwamoto, E.L. Effects of α-pinene on the mitochondrial respiration of maize seedlings. Plant. Physiol. Biochem. 2003, 41, 985–991. [Google Scholar] [CrossRef]
- Mucciarelli, M.; Camusso, W.; Bertea, C.M.; Maffei, M. Effect of (+)-pulegone and other oil components of Mentha x Piperita on cucumber respiration. Phytochemistry 2001, 57, 91–98. [Google Scholar] [CrossRef]
- Weir, T.L.; Park, S.-W.; Vivanco, J.M. Biochemical and physiological mechanisms mediated by allelochemicals. Curr. Opin. Plant. Biol. 2004, 7, 472–479. [Google Scholar] [CrossRef]
- Luiza Ishii-Iwamoto, E.; Marusa Pergo Coelho, E.; Reis, B.; Sebastiao Moscheta, I.; Moacir Bonato, C. Effects of monoterpenes on physiological processes during seed germination and seedling growth. Curr. Bioact. Compd. 2012, 8, 50–64. [Google Scholar] [CrossRef]
- Lorber, P.; Muller, W.H. Volatile growth inhibitors produced by Salvia leucophylla: Effects on seedling root tip ultrastructure. Am. J. Bot. 1976, 63, 196–200. [Google Scholar] [CrossRef]
- Yoshimura, H.; Sawai, Y.; Tamotsu, S.; Sakai, A. 1,8-cineole inhibits both proliferation and elongation of BY-2 cultured tobacco cells. J. Chem. Ecol. 2011, 37, 320–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atsushi, S.; Hiroko, Y. Monoterpenes of salvia leucophylla. Curr. Bioact. Compd. 2012, 8, 90–100. [Google Scholar] [CrossRef]
- Chaimovitsh, D.; Abu-Abied, M.; Belausov, E.; Rubin, B.; Dudai, N.; Sadot, E. Microtubules are an intracellular target of the plant terpene citral. Plant. J. 2010, 61, 399–408. [Google Scholar] [CrossRef]
- Chaimovitsh, D.; Stelmakh, O.R.; Altshuler, O.; Belausov, E.; Abu-Abied, M.; Rubin, B.; Sadot, E.; Dudai, N. The relative effect of citral on mitotic microtubules in wheat roots and BY2 cells. Plant. Biol. 2012, 14, 354–364. [Google Scholar] [CrossRef]
- Grana, E.; Sotelo, T.; Díaz-Tielas, C.; Araniti, F.; Krasuska, U.; Bogatek, R.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Citral induces auxin and ethylene-mediated malformations and arrests cell division in Arabidopsis thaliana roots. J. Chem. Ecol. 2013, 39, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Chaimovitsh, D.; Shachter, A.; Abu-Abied, M.; Rubin, B.; Sadot, E.; Dudai, N.; Shechter, A.; Abu-Abeid, M. Herbicidal activity of monoterpenes is associated with disruption of microtubule functionality and membrane integrity. Weed Sci. 2016, 65, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Sarheed, M.M.; Rajabi, F.; Kunert, M.; Boland, W.; Wetters, S.; Miadowitz, K.; Kaźmierczak, A.; Sahi, V.P.; Nick, P. Cellular base of mint allelopathy: Menthone affects plant microtubules. Front. Plant Sci. 2020, 11, 1320. [Google Scholar]
- Pawlowski, A.; Kaltchuk-Santos, E.; Zini, C.A.; Caramão, E.; Soares, G. Essential oils of Schinus terebinthifolius and S. molle (Anacardiaceae): Mitodepressive and aneugenic inducers in onion and lettuce root meristems. S. Afr. J. Bot. 2012, 80, 96–103. [Google Scholar] [CrossRef]
- Fagodia, S.K.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phytotoxicity and cytotoxicity of Citrus aurantiifolia essential oil and its major constituents: Limonene and citral. Ind. Crop. Prod. 2017, 108, 708–715. [Google Scholar] [CrossRef]
- Pinheiro, P.F.; Costa, A.V.; Alves, T.D.A.; Galter, I.N.; Pinheiro, C.A.; Pereira, A.F.; Oliveira, C.M.R.; Fontes, M.M.P. Phytotoxicity and cytotoxicity of essential oil from leaves of Plectranthus amboinicus, carvacrol, and thymol in plant bioassays. J. Agric. Food Chem. 2015, 63, 8981–8990. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singh, H.P.; Batish, D.R.; Kohli, R.K.; Yadav, S.S. Chemical characterization, phytotoxic, and cytotoxic activities of essential oil of Mentha longifolia. Environ. Sci. Pollut. Res. 2020, 27, 13512–13523. [Google Scholar] [CrossRef] [PubMed]
- Bozari, S.; Agar, G.; Aksakal, O.; Erturk, F.A.; Yanmis, D. Determination of chemical composition and genotoxic effects of essential oil obtained from Nepeta nuda on Zea mays seedlings. Toxicol. Ind. Health 2013, 29, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Issa, M.; Chandel, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K.; Yadav, S.S.; Kumari, A. Appraisal of phytotoxic, cytotoxic and genotoxic potential of essential oil of a medicinal plant Vitex negundo. Ind. Crop. Prod. 2020, 145, 112083. [Google Scholar] [CrossRef]
- Oosterhaven, K.; Hartmans, K.J.; Huizing, H.J. Inhibition of potato (Solanum tuberosum) sprout growth by the monoterpene S-carvone: Reduction of 3-hydroxy-3-methylglutaryl coenzyme a reductase activity without effect on its mRNA level. J. Plant. Physiol. 1993, 141, 463–469. [Google Scholar] [CrossRef]
- Oosterhaven, K.; Hartmans, K.J.; Scheffer, J.J.C. Inhibition of potato sprout growth by carvone enantiomers and their bioconversion in sprouts. Potato Res. 1995, 38, 219–230. [Google Scholar] [CrossRef]
- Rentzsch, S.; Podzimska, D.; Voegele, A.; Imbeck, M.; Müller, K.; Linkies, A.; Leubner-Metzger, G. Dose- and tissue-specific interaction of monoterpenes with the gibberellin-mediated release of potato tuber bud dormancy, sprout growth and induction of α-amylases and β-amylases. Planta 2012, 235, 137–151. [Google Scholar] [CrossRef]
- Romagni, J.G. Inhibition of plant asparagine synthetase by monoterpene cineoles. Plant. Physiol. 2000, 123, 725–732. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, K.; Hutzler, J.; Tresch, S.; Christiansen, N.; Looser, R.; Ehrhardt, T. On the mode of action of the herbicides cinmethylin and 5-benzyloxymethyl-1, 2-isoxazolines: Putative inhibitors of plant tyrosine aminotransferase. Pest. Manag. Sci. 2011, 68, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Abdelgaleil, S.A.M.; Gouda, N.A.; Saad, M.M.G. Phytotoxic properties of monoterpenes on Silybum marianum (L.) gaertn. and their inhibitory effect on carbonic anhydrase activity. Aust. J. Basic Appl. Sci. 2014, 8, 356–363. [Google Scholar]
- Araniti, F.; Bruno, L.; Sunseri, F.; Pacenza, M.; Forgione, I.; Bitonti, M.B.; Abenavoli, M.R. The allelochemical farnesene affects Arabidopsis thaliana root meristem altering auxin distribution. Plant. Physiol. Biochem. 2017, 121, 14–20. [Google Scholar] [CrossRef]
- Massimo, E. Maffei monoterpenoid plant-plant interactions upon herbivory. Curr. Bioact. Compd. 2012, 8, 65–70. [Google Scholar] [CrossRef]
- Sukegawa, S.; Shiojiri, K.; Higami, T.; Suzuki, S.; Arimura, G. ichiro Pest management using mint volatiles to elicit resistance in soy: Mechanism and application potential. Plant J. 2018, 96, 910–920. [Google Scholar] [CrossRef] [Green Version]
- Banani, H.; Olivieri, L.; Santoro, K.; Garibaldi, A.; Gullino, M.L.; Spadaro, D. Thyme and savory essential oil efficacy and induction of resistance against botrytis cinerea through priming of defense responses in apple. Foods 2018, 7, 11. [Google Scholar] [CrossRef] [Green Version]
- Godard, K.A.; White, R.; Bohlmann, J. Monoterpene-induced molecular responses in Arabidopsis thaliana. Phytochemistry 2008, 69, 1838–1849. [Google Scholar] [CrossRef]
- Vergnes, S.; Ladouce, N.; Fournier, S.; Ferhout, H.; Attia, F.; Dumas, B. Foliar treatments with Gaultheria procumbens essential oil induce defense responses and resistance against a fungal pathogen in Arabidopsis. Front. Plant. Sci. 2014, 5, 477. [Google Scholar] [CrossRef] [PubMed]
- Ben-Jabeur, M.; Ghabri, E.; Myriam, M.; Hamada, W. Thyme essential oil as a defense inducer of tomato against gray mold and Fusarium wilt. Plant. Physiol. Biochem. 2015, 94, 35–40. [Google Scholar] [CrossRef]
- Perina, F.J.; De Andrade, C.C.L.; Moreira, S.I.; Nery, E.M.; Ogoshi, C.; Alves, E. Cinnamomun zeylanicum oil and trans-cinnamaldehyde against Alternaria brown spot in tangerine: Direct effects and induced resistance. Phytoparasitica 2019, 47, 575–589. [Google Scholar] [CrossRef]
- Lucas, G.C.; Alves, E.; Pereira, R.B.; Zacaroni, A.B.; Perina, F.J.; de Souza, R.M. Indian clove essential oil in the control of tomato bacterial spot. J. Plant Pathol. 2012, 94, 45–51. [Google Scholar]
- Borges Pereira, R.; Lucas, G.C.; Perina, J.; Martins, P.; Júnior, R.; Alves, E. Citronella essential oil in the control and activation of coffee plants defense response agains rust and brown eye spot. Cienc. Agrotec. 2012, 36, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Matsui, K.; Sugimoto, K.; Mano, J.; Ozawa, R.; Takabayashi, J. Differential metabolisms of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PLoS ONE 2012, 7, e36433. [Google Scholar] [CrossRef] [PubMed]
- Vaněk, T.; Novotný, M.; Podlipná, R.; Šaman, D.; Valterová, I. Biotransformation of citronellal by Solanum aviculare suspension cultures: Preparation of p-menthane-3,8-diols and determination of their absolute configurations. J. Nat. Prod. 2003, 66, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Dudai, N.; Larkov, O.; Putievsky, E.; Lerner, H.R.; Ravid, U.; Lewinsohn, E.; Mayer, A.M. Biotransformation of constituents of essential oils by germinating wheat seed. Phytochemistry 2000, 55, 375–382. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Nunes, I.S.; Figueiredo, A.C.; Pedro, L.G.; Trindade, H.; Barroso, J.G. Biotransformation of menthol and geraniol by hairy root cultures of Anethum graveolens: Effect on growth and volatile components. Biotechnol. Lett. 2009, 31, 897–903. [Google Scholar] [CrossRef]
- Rivas, F.; Parra, A.; Martinez, A.; García-Granados, A. Enzymatic glycosylation of terpenoids. Phytochem. Rev. 2013, 12, 327–339. [Google Scholar] [CrossRef]
- Shimoda, K.; Kondo, Y.; Nishida, T.; Hamada, H.; Nakajima, N.; Hamada, H. Biotransformation of thymol, carvacrol, and eugenol by cultured cells of Eucalyptus perriniana. Phytochemistry 2006, 67, 2256–2261. [Google Scholar] [CrossRef]
- Orihara, Y.; Furuya, T. Biotransformation of 1,8-cineole by cultured cells of Eucalyptus perriniana. Phytochemistry 1994, 35, 641–644. [Google Scholar] [CrossRef]
- Orihara, Y.; Furuya, T. Biotransformation of (+)- and (−)-fenchone by cultured cells of Eucalyptus perriniana. Phytochemistry 1994, 36, 55–59. [Google Scholar] [CrossRef]
- Shimoda, K.; Ishimoto, H.; Kamiue, T.; Kobayashi, T.; Hamada, H.; Hamada, H. Glycosylation of sesamol by cultured plant cells. Phytochemistry 2009, 70, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.; Eshita, Y.; Katsuragi, H.; Hamada, H.; Shimoda, K.; Kubota, N. Glycosylation of vanillin and 8-nordihydrocapsaicin by cultured Eucalyptus perriniana cells. Molecules 2012, 17, 5013–5020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, A.C.; Scheffer, J.J.C. Biotransformation of monoterpenes and sesquiterpenes by cell suspension cultures of Achillea millefolium L. ssp. millefolium. Biotechnol. Lett. 1996, 18, 863–868. [Google Scholar] [CrossRef]
- Sugimoto, K.; Matsui, K.; Takabayashi, J. Conversion of volatile alcohols into their glucosides in Arabidopsis. Commun. Integr. Biol. 2015, 8, 992731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamauchi, Y.; Kunishima, M.; Mizutani, M.; Sugimoto, Y. Reactive short-chain leaf volatiles act as powerful inducers of abiotic stress-related gene expression. Sci. Rep. 2015, 5, 8030. [Google Scholar] [CrossRef] [Green Version]
- Muramoto, S.; Matsubara, Y.; Mwenda, C.M.; Koeduka, T.; Sakami, T.; Tani, A.; Matsui, K. Glutathionylation and reduction of methacrolein in tomato plants account for its absorption from the vapor phase. Plant. Physiol. 2015, 169, 1744–1754. [Google Scholar] [CrossRef] [Green Version]
- Galindo, J.C.G.; Hernández, A.; Dayan, F.E.; Tellez, M.R.; Macías, F.A.; Paul, R.N.; Duke, S.O. Dehydrozaluzanin C, a natural sesquiterpenolide, causes rapid plasma membrane leakage. Phytochemistry 1999, 52, 805–813. [Google Scholar] [CrossRef]
- Synowiec, A.; Możdżeń, K.; Krajewska, A.; Landi, M.; Araniti, F. Carum carvi L. essential oil: A promising candidate for botanical herbicide against Echinochloa crus-galli (L.) P. Beauv. in maize cultivation. Ind. Crop. Prod. 2019, 140, 111652. [Google Scholar] [CrossRef]
- Araniti, F.; Miras-Moreno, B.; Lucini, L.; Landi, M.; Abenavoli, M.R. Metabolomic, proteomic and physiological insights into the potential mode of action of thymol, a phytotoxic natural monoterpenoid phenol. Plant. Physiol. Biochem. 2020, 153, 141–153. [Google Scholar] [CrossRef]
- Rienth, M.; Crovadore, J.; Ghaffari, S.; Lefort, F. Oregano essential oil vapour prevents Plasmopara viticola infection in grapevine (Vitis Vinifera) and primes plant immunity mechanisms. PLoS ONE 2019, 14, e0222854. [Google Scholar] [CrossRef] [PubMed]
- Brokl, M.; Fauconnier, M.L.; Benini, C.; Lognay, G.C.; Du Jardin, P.; Focant, J.F. Improvement of ylang-ylang essential oil characterization by GC×GC-TOFMS. Molecules 2013, 18, 1783–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benini, C.; Ringuet, M.; Wathelet, J.P.; Lognay, G.C.; Jardin, P.; Fauconnier, M.L. Variations in the essential oils from ylang-ylang (Cananga odorata [Lam.] Hook f. & Thomson forma genuina) in the Western Indian Ocean islands. Flavour Fragr. J. 2012, 27, 356–366. [Google Scholar] [CrossRef]
- Tanoh, E.A.; Boué, G.B.; Nea, F.; Genva, M.; Wognin, E.L.; LeDoux, A.; Martin, H.; Tonzibo, F.Z.; Frédérich, M.; Fauconnier, M.L. Seasonal effect on the chemical composition, insecticidal properties and other biological activities of Zanthoxylum leprieurii guill. & perr. essential oils. Foods 2020, 9, 550. [Google Scholar] [CrossRef]
- Nea, F.; Tanoh, E.A.; Wognin, E.L.; Kemene, T.K.; Genva, M.; Saive, M.; Tonzibo, Z.F.; Fauconnier, M.L. A new chemotype of Lantana rhodesiensis Moldenke essential oil from Côte d’Ivoire: Chemical composition and biological activities. Ind. Crop. Prod. 2019, 141, 111766. [Google Scholar] [CrossRef]
- Mirmostafaee, S.; Azizi, M.; Fujii, Y. Study of allelopathic interaction of essential oils from medicinal and aromatic plants on seed germination and seedling growth of lettuce. Agronomy 2020, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Souri, M.K.; Bakhtiarizade, M. Biostimulation effects of rosemary essential oil on growth and nutrient uptake of tomato seedlings. Sci. Hortic. 2019, 243, 472–476. [Google Scholar] [CrossRef]
- Hara, M. Potential use of essential oils to enhance heat tolerance in plants. Z. Naturforsch. Sect. C J. Biosci. 2020, 75, 225–231. [Google Scholar] [CrossRef]
- Calabrese, E.J. The emergence of the dose–Response concept in biology and medicine. Int. J. Mol. Sci. 2016, 17, 2034. [Google Scholar] [CrossRef] [Green Version]
- Uthappa, U.T.; Kurkuri, M.D.; Kigga, M. Nanotechnology Advances for the Development of Various Drug Carriers. In Nanobiotechnology in Bioformulations; Springer: Cham, Switzerland, 2019; pp. 187–224. [Google Scholar]
- Maes, C.; Bouquillon, S.; Fauconnier, M.L. Maes Encapsulation of essential oils for the development of biosourced pesticides with controlled release: A review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef] [Green Version]
- Villaverde, J.J.; Sevilla-Morán, B.; Sandín-España, P.; López-Goti, C.; Alonso-Prados, J.L. Biopesticides in the framework of the European Pesticide Regulation (EC) No. 1107/2009. Pest Manag. Sci. 2014, 70, 2–5. [Google Scholar] [CrossRef]
- Cloyd, R.A.; Galle, C.L.; Keith, S.R.; Kalscheur, N.A.; Kemp, K.E. Effect of commercially available plant-derived essential oil products on arthropod pests. J. Econ. Entomol. 2009, 102, 1567–1579. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, N.G. Medical Toxicology of Natural Substances: Foods, Fungi, Medicinal Herbs, Plants, and Venomous Animals; Wiley: Hoboken, NJ, USA, 2008; pp. 1–1157. [Google Scholar]
- Environmental Protection Agency. US EPA—Pesticides—Fact Sheet for Oil of Citronella. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/fs_PC-021901_1-Feb-97.pdf?rel=outbound (accessed on 5 September 2020).
- Mishra, J.; Tewari, S.; Singh, S.; Arora, N.K. Biopesticides: Where we stand? In Plant Microbes Symbiosis: Applied Facets; Springer: New Delhi, India, 2015; pp. 37–75. [Google Scholar]


| Mode of Action | Essential Oils or Constituents (Concentration) | Application Mode (Time) | Plant Target | Observation | Ref |
|---|---|---|---|---|---|
| Water status alteration | Camphor (10 mg/L) menthol (5 mg/L) | Vapor exposure (for 24 to 96 h) | A. thaliana | Scanning electron microscopy, transpiration, PCR, western blot | [25] |
| Camphor (10 mg/L) | Vapor exposure (for 24 to 96 h) | A. thaliana | Real time PCR, in vivo cytoskeleton visualization | [26] | |
| Clove oil (2.5%) eugenol (1.5%) | Sprayed at 50 mL/m2 | Broccoli, lambsquarte, pigweed | Membrane integrity (EL), spray solution retention | [27] | |
| Citral (1200–2400 μM) | Watered every 2 day (25 mL per pot) | A. thaliana | Water/osmotic potentials (Ψw/Ψs), pigment, protein, anthocyanin, stomata density | [28] | |
| Trans-caryophyllene (450–1800 µM) | Watering (25 mL/pot) or spraying (15 mL/pot) | A. thaliana | Chlorophyll a fluorescence, osmotic potential, MDA, pigment, proline, protein and element content | [29] | |
| Membrane properties and interaction | Mentha piperita (5–900 ppm) | Perfusion | Cucumis sativus | Root segment membrane potential determination | [31] |
| C. zeylanicym C. winterianus (3%) | Sprayed (10 L/m2) | A. thaliana | Herbicide tests + in silico approach | [35] | |
| 1,8-cineole, thymol, menthol, geraniol, camphor (21.7, 2.0, 1.9, 2.5, 7.4 mg/L) | Vapor exposure | Zea mays | Lipid, peroxide and lipid peroxidation | [36] | |
| Sterols and phospholipid fatty acid (PLFA) composition | [37] | ||||
| Reactive oxygen and nitrogen species induction | α-pinene (1.36–136 mg/mL) | Vapor exposure in petri dish for 3, 5 and 7 days | C. occidentalis, A. viridis, T. aestivum, Pisum sativum, Cicer arietinum | EL, MDA, H2O2, proline, ROS scavenging enzymes (SOD, APX, GPX, CAT, GR) | [50] |
| β-Pinene (0.02–0.80 mg/mL) | [42] | ||||
| β-pinene (1.36–13.6 µg/mL) | Vapor exposure for 4 to 24 h | Wheat seed | H2O2, O2−, MDA, ROS scavenging enzymes, LOX | [43] | |
| Citronellol (50–250 μM) | Watered for 24, 48 and 72 h | Wheat seed | MDA, EL, CDs, LOX, In situ histochemical analyses | [44] | |
| P. benghalensis (0.25–2.5 mg/mL) | Vapor exposure | Avena fatua Phalaris minor | H2O2, O2−, MDA, CDs, EL, ROS scavenging enzymes | [45] | |
| Monarda didyma (0.06–1.25 µg/mL) | Vapor exposure for 5 days | Weed seed | H2O2, MDA | [46] | |
| Artemisia scoparia (0.14–0.70 mg/mL) | Vapor exposure for 5 days | Wheat seed | O2−, H2O2, proline, root oxidizability, cell death | [47] | |
| Heterothalamus psiadioides (1–5 µL) | Vapor exposure in petri dish for 7 days | A. thaliana | Histochemical detection of H2O2 | [48] | |
| Photosynthesis inhibition | β-pinene (135 µM) | Applied to organelles suspension | Chloroplast (Spinacia oleracea) | O2, protein, chlorophyll, electron microscopy | [51] |
| β-pinene (945 µM) | Applied to organelles suspension | Chloroplast (Cucurbita pepo) | O2, protein, chlorophyll, Gel electrophoresis and immunoblotting | [52] | |
| β-pinene (0.02–0.80 mg/mL) | Vapor exposure for 3, 5 and 7 days | Oryza sativa | Chlorophyll, protein, carbohydrate, proteases, α- and β-amylases, POD, PER | [53] | |
| Cymbopogon citratus (1.25–10% (v/v)) | Foliar sprayed at 1000 L ha−1 | Barnyardgrass | Chlorophyll a, b and carotenoid, EL, MDA | [54] | |
| Photosynthesis inhibition | Hyptis suaveolens (1–5% (v/v)) | Foliar sprayed (10 mL/plant) | Oryza sativaE. crus-galli | Total chlorophyll content, cell viability, Cytogenetic analysis | [55] |
| Farnesene (0–1200 μM) | Grown in medium for 14 days | A. thaliana | Root gravitropism, structural studies, electron microscopy, O2−, H2O2, microtubule, ethylene, auxin | [56] | |
| Artemisia fragrans (0.5, 1, 2 and 4%) | Spraying (100 mL/ pot) for 5 days | Convolvulus arvensis | Chlorophyll a fluorescence, chlorophyll, ROS scavenging enzymes, H2O2, MDA | [57] | |
| Clove oil (2.5%), eugenol (1.95%) | Covered by solutions | Broccoli | Chlorophyll a fluorescence imaging at 20, 40 and 60 min | [58] | |
| Origanum vulgare (0–500 μL/L) | Grown in medium for 10 days | A. thaliana | Chlorophyll a fluorescence, chlorophyll, protein, MDA, Ionomic, metabolomic | [59] | |
| Mitochondrial respiration inhibition | 1,8-cineole (6 mM) | Apply to organelle | A. fatua | O2 consumption | [60] |
| Juglone (10 mM) | Bathed in dark for 30 min | Soybean cotyledons | O2 consumption and isotope fractionation | [61] | |
| Mitochondrial respiration inhibition | α-pinene, camphor, eucalyptol and limonene (0.1–10 mM) | Vapor exposure/apply to organelle | Maize | Protein, seed germination, growth test and oxygen uptake | [62] |
| α– pinene (50–500 µM) | Grown in medium for 10 days | Coleoptiles and primary roots of maize | O2 consumption, mitochondrial ATP production | [63] | |
| Pulegone, menthol, menthone (0–1500 ppm) | Foliar sprayed | Cucumber seeds (roots segments, mitochondria) | O2 uptake, mitochondrial respiration | [64] | |
| Camphor, 1,8-Cineole, Limonene, α–pinene (0–500 µM) | Apply to organelle suspension | Corn and soybean | Mitochondrial respiration | [66] | |
| 1,8-cineole (0–2000 µM) | Vapor exposure | N. tabacum (seeds) | Growth, protoplasts proliferation, starch accumulation of BY-2 | [68] | |
| Microtubule disruption and genotoxicity | Citral (0–1.0 μL) | Vapor exposure | A. thaliana | Microscopy, in vitro polymerization of microtubules | [70] |
| Citral (0–1.200 μM) | Grown inmedium for 14 days | A. thaliana | Ultra-structural, pectin and callose staining, mitotic indices, ethylene, auxin | [71] | |
| Limonene, citral, carvacrol, pulegone (4.6–9.2 μmol/20 mL) | Vapor exposure for 0, 15, 30 and 60 min | A. thaliana | Membrane, microtubules, F-actin, (confocal microscopy), in Planta monoterpene concentrations | [73] | |
| Menthone | Vapor exposure | Tobacco BY-2A. thaliana | GFP-tagged markers for microtubules and actin filaments | [74] | |
| Schinus molle Schinus terebinthifolius | Vapor exposure 0.1 mL for 72 h | Allium cepa, Lactuca sativa | Cytogenetic assay | [75] | |
| Citrus aurantiifolia (0.10–1.50 mg/mL) | Vapor exposure (10 mL) for 3–24 h | Avena fatua, E. crus-galli, Phalaris minor | Phytotoxicity: dose-response assay, cytotoxicity (Allium cepa) | [76] | |
| Plectrantus amboinicus (0–0.120% w/v) | Vapor exposure for 48 h | Lactuca sativa Sorghum bicolor | Germination speed index, percentage of germination | [77] | |
| Mentha longifolia (10–250 μg/mL) (0.5–5%) | Vapor exposure Foliar sprayed (5 mL/pot) | Cyperus rotundus, E.crus-galli, Oryza sativa | Germination, root length, coleoptile length, chlorophyll, cytotoxicity assay (Allium cepa) | [78] | |
| Microtubule disruption and genotoxicity | Nepeta nuda (0.1–0.8 µL/mL) | Vapor exposure (10 mL) for 7 days | Zea mays | Randomly amplified polymorphic DNA, quantitative analysis of proteins | [79] |
| Salvia leucophylla (0–1300 µM) | Vapor exposure for 4 days | Brassica campestris | DAPI-fluorescence microscopy, immunofluorescence microscopy, DNA Synthesis Activities | [80] | |
| Vitex negundo (0.1–2.5 mg/mL) | Vapor exposure (12 mL) | Avena Fatua, E. crus-galli, Onion bulbs | Phytotoxicity, cytoxicity | [81] | |
| S-carvone (125 µL) | Vapor exposure (several days) | Solanum tuberosum | Potato sprout growth, HMGR activity, membrane protein composition, transcription activity | [82] | |
| Phytohormones | R/S-carvone (25–125 µL) | Vapor exposure (several days) | Solanum tuberosum | Growth inhibition, carvone and conversion products in potato sprouts | [83] |
| Peppermint oil (0.1% (v/v)) | Vapor exposure | Solanum tuberosum | Potato sprout growth, protein extraction, enzyme activity, semi quantitative RT-PCR for potato α–amylase | [84] | |
| Ten monoterpenes (0.5–2 mM) | Vapor exposure (6 mL) for 9 days | Silybum marianum | carbonic anhydrase activity | [87] | |
| Farnesene (250 μM) | Grown in medium for 14 days | A. thaliana | Root anatomy/meristem size, mitotic indices, quantitative PCR, auxin gradient and polar transport | [88] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werrie, P.-Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.-L. Phytotoxicity of Essential Oils: Opportunities and Constraints for the Development of Biopesticides. A Review. Foods 2020, 9, 1291. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9091291
Werrie P-Y, Durenne B, Delaplace P, Fauconnier M-L. Phytotoxicity of Essential Oils: Opportunities and Constraints for the Development of Biopesticides. A Review. Foods. 2020; 9(9):1291. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9091291
Chicago/Turabian StyleWerrie, Pierre-Yves, Bastien Durenne, Pierre Delaplace, and Marie-Laure Fauconnier. 2020. "Phytotoxicity of Essential Oils: Opportunities and Constraints for the Development of Biopesticides. A Review" Foods 9, no. 9: 1291. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9091291