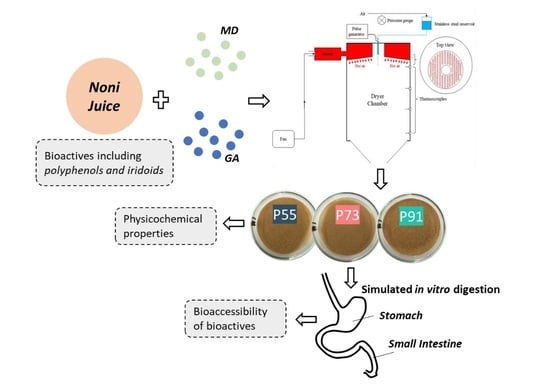
Fabrication of Spray-Dried Microcapsules Containing Noni Juice Using Blends of Maltodextrin and Gum Acacia: Physicochemical Properties of Powders and Bioaccessibility of Bioactives during In Vitro Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Feed Solution Preparation
2.3. Spray-Drying Process
2.4. Morphological Observation
2.5. Moisture Content and Water Activity
2.6. Bulk Density and Tapped Density
2.7. Dissolution Rate
2.8. Glass Transition Temperature
2.9. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.10. Determination of Bioactive Compounds
2.10.1. Total Phenolic Content (TPC)
2.10.2. Total Antioxidant Activity
2.10.3. Deacetylasperulosidic Acid (DAA) and Asperulosidic Acid (AA) Content
2.11. In Vitro Digestion Study
2.12. Statistical Analysis
3. Results and Discussion
3.1. Microstructure of FNJ Microcapsules
3.2. Physicochemical Properties
3.2.1. Particle Size Distribution
3.2.2. Moisture Content and Water Activity
3.2.3. Bulk and Tapped Density
3.2.4. Dissolution Rate
3.2.5. Glass Transition Temperature
3.2.6. ATR-FTIR Characterisation
3.3. Bioactive Stability during In Vitro Digestion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Abou Assi, R.; Darwis, Y.; Abdulbaqi, I.M.; Vuanghao, L.; Laghari, M.H. Morinda citrifolia (Noni): A comprehensive review on its industrial uses, pharmacological activities, and clinical trials. Arab. J. Chem. 2017, 10, 691–707. [Google Scholar]
- Pawlus, A.D.; Kinghorn, A.D. Review of the ethnobotany, chemistry, biological activity and safety of the botanical dietary supplement Morinda citrifolia (noni). J. Pharm. Pharm. 2007, 59, 1587–1609. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Debnath, S.; Harigaya, Y. Naturally occurring secoiridoids and bioactivity of naturally occurring iridoids and secoiridoids. A review, part 2. Chem. Pharm. Bull. 2007, 55, 689–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Zhang, C.; Khoo, S.L.A.; Chen, X.D.; Quek, S.Y. Microencapsulation of fermented noni juice via micro-fluidic-jet spray drying: Evaluation of powder properties and functionalities. Powder Technol. 2020, 361, 995–1005. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Zhang, J.; Kilmartin, P.A.; Quek, S.Y. Exploring the effects of microencapsulation on odour retention of fermented noni juice. J. Food Eng. 2020, 273, 109892. [Google Scholar] [CrossRef]
- Sarabandi, K.; Peighambardoust, S.H.; Mahoonak, A.S.; Samaei, S.P. Effect of carrier types and compositions on the production yield, microstructure and physical characteristics of spray dried sour cherry juice concentrate. J. Food Meas. Charact. 2017, 11, 1602–1612. [Google Scholar] [CrossRef]
- Frascareli, E.C.; Silva, V.M.; Tonon, R.V.; Hubinger, M.D. Effect of process conditions on the microencapsulation of coffee oil by spray drying. Food Bioprod. Process. 2012, 90, 413–424. [Google Scholar] [CrossRef]
- Zhang, C.; Quek, S.Y.; Fu, N.; Liu, B.; Kilmartin, P.A.; Chen, X.D. A study on the structure formation and properties of noni juice microencapsulated with maltodextrin and gum acacia using single droplet drying. Food Hydrocoll. 2019, 88, 199–209. [Google Scholar] [CrossRef]
- Kanakdande, D.; Bhosale, R.; Singhal, R.S. Stability of cumin oleoresin microencapsulated in different combination of gum arabic, maltodextrin and modified starch. Carbohydr. Polym. 2007, 67, 536–541. [Google Scholar] [CrossRef]
- Krishnan, S.; Bhosale, R.; Singhal, R.S. Microencapsulation of cardamom oleoresin: Evaluation of blends of gum arabic, maltodextrin and a modified starch as wall materials. Carbohydr. Polym. 2005, 61, 95–102. [Google Scholar] [CrossRef]
- Fernandes, L.P.; Candido, R.C.; Oliveira, W.P. Spray drying microencapsulation of Lippia sidoides extracts in carbohydrate blends. Food Bioprod. Process. 2012, 90, 425–432. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Quantitative analysis, in vitro assessment of bioavailability and antioxidant activity of food carotenoids—A review. J. Food Compost. Anal. 2010, 23, 726–740. [Google Scholar] [CrossRef]
- Garrett, D.A.; Failla, M.L.; Sarama, R.J. Development of an in vitro digestion method to assess carotenoid bioavailability from meals. J. Agric. Food Chem. 1999, 47, 4301–4309. [Google Scholar] [CrossRef] [PubMed]
- Granado-Lorencio, F.; Olmedilla-Alonso, B.; Herrero-Barbudo, C.; Pérez-Sacristán, B.; Blanco-Navarro, I.; Blázquez-García, S. Comparative in vitro bioaccessibility of carotenoids from relevant contributors to carotenoid intake. J. Agric. Food Chem. 2007, 55, 6387–6394. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A. Influence of food matrix on the bioaccessibility of fruit polyphenolic compounds. J. Agric. Food Chem. 2020, 68, 1315–1325. [Google Scholar] [CrossRef]
- Cai, Y.; Qin, W.; Ketnawa, S.; Ogawa, Y. Impact of particle size of pulverized citrus peel tissue on changes in antioxidant properties of digested fluids during simulated in vitro digestion. Food Sci. Hum. Well. 2019, 9, 58–63. [Google Scholar] [CrossRef]
- Dalmau, M.E.; Eim, V.; Rosselló, C.; Cárcel, J.A.; Simal, S. Effects of convective drying and freeze-drying on the release of bioactive compounds from beetroot during in vitro gastric digestion. Food Funct. 2019, 10, 3209–3223. [Google Scholar] [CrossRef]
- Ketnawa, S.; Suwannachot, J.; Ogawa, Y. In vitro gastrointestinal digestion of crisphead lettuce: Changes in bioactive compounds and antioxidant potential. Food Chem. 2020, 311, 125885. [Google Scholar] [CrossRef]
- Pavan, V.; Sancho, R.A.S.; Pastore, G.M. The effect of in vitro digestion on the antioxidant activity of fruit extracts (Carica papaya, Artocarpus heterophillus and Annona marcgravii). LWT Food Sci. Technol. 2014, 59, 1247–1251. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Quek, S.Y.; Fu, N.; Su, Y.; Kilmartin, P.A.; Chen, X.D. Storage stability and in vitro digestion of microencapsulated powder containing fermented noni juice and probiotics. Food Biosci. 2020, 37, 100740. [Google Scholar] [CrossRef]
- Omobuwajo, T.; Busari, O.; Osemwegie, A. Thermal agglomeration of chocolate drink powder. J. Food Eng. 2000, 46, 73–81. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Zhang, B.; Chen, P.X.; Liu, R.; Tsao, R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.G.; Bishop, K.S.; Tanambell, H.; Buchanan, P.; Quek, S.Y. Assessment of in vitro bioactivities of polysaccharides isolated from Hericium Novae-Zealandiae. Antioxidants 2019, 8, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Amelia, R.; Wu, W.D.; Cashion, J.; Bao, P.; Zheng, R.; Chen, X.D.; Selomulya, C. Microfluidic spray drying as a versatile assembly route of functional particles. Chem. Eng. Sci. 2011, 66, 5531–5540. [Google Scholar] [CrossRef]
- Walton, D. The morphology of spray-dried particles a qualitative view. Dry. Technol. 2000, 18, 1943–1986. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of grape polyphenols using maltodextrin and gum arabic as two alternative coating materials: Development and characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Dellarosa, N.; Tylewicz, U.; Laghi, L.; Romani, S.; Dalla Rosa, M.; Witrowa-Rajchert, D. The influence of carrier material on some physical and structural properties of carrot juice microcapsules. Food Chem. 2017, 236, 134–141. [Google Scholar] [CrossRef]
- Shi, Q.; Fang, Z.; Bhandari, B. Effect of addition of whey protein isolate on spray-drying behavior of honey with maltodextrin as a carrier material. Dry. Technol. 2013, 31, 1681–1692. [Google Scholar] [CrossRef] [Green Version]
- Fu, N.; Zhou, Z.; Jones, T.B.; Tan, T.T.; Wu, W.D.; Lin, S.X.; Chen, X.D.; Chan, P.P.Y. Production of monodisperse epigallocatechin gallate (EGCG) microparticles by spray drying for high antioxidant activity retention. Int. J. Pharm. 2011, 413, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fu, N.; Quek, S.Y.; Zhang, J.; Chen, X.D. Exploring the drying behaviors of microencapsulated noni juice using reaction engineering approach (REA) mathematical modelling. J. Food Eng. 2019, 248, 53–61. [Google Scholar] [CrossRef]
- Ramakrishnan, Y.; Adzahan, N.M.; Yusof, Y.A.; Muhammad, K. Effect of wall materials on the spray drying efficiency, powder properties and stability of bioactive compounds in tamarillo juice microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Carneiro, H.C.; Tonon, R.V.; Grosso, C.R.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- Fernandes, R.V.d.B.; Borges, S.V.; Botrel, D.A. Influence of spray drying operating conditions on microencapsulated rosemary essential oil properties. Food Sci. Technol. 2013, 33, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Bicudo, M.O.P.; Jó, J.; Oliveira, G.A.D.; Chaimsohn, F.P.; Sierakowski, M.R.; Freitas, R.A.D.; Ribani, R.H. Microencapsulation of juçara (Euterpe edulis M.) pulp by spray drying using different carriers and drying temperatures. Dry. Technol. 2015, 33, 153–161. [Google Scholar] [CrossRef]
- Kingwatee, N.; Apichartsrangkoon, A.; Chaikham, P.; Worametrachanon, S.; Techarung, J.; Pankasemsuk, T. Spray drying Lactobacillus casei 01 in lychee juice varied carrier materials. LWT-Food Sci. Technol. 2015, 62, 847–853. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Assadpoor, E.; Dehnad, D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int. J. Biol. Macromol. 2016, 85, 379–385. [Google Scholar] [CrossRef]
- Vardin, H.; Yasar, M. Optimisation of pomegranate (Punica Granatum L.) juice spray-drying as affected by temperature and maltodextrin content. Int. J. Food Sci. Technol. 2012, 47, 167–176. [Google Scholar] [CrossRef]
- Finney, J.; Buffo, R.; Reineccius, G. Effects of type of atomization and processing temperatures on the physical properties and stability of spray-dried flavors. J. Food Sci. 2002, 67, 1108–1114. [Google Scholar] [CrossRef]
- Yousefi, S.; Emam-Djomeh, Z.; Mousavi, S. Effect of carrier type and spray drying on the physicochemical properties of powdered and reconstituted pomegranate juice (Punica Granatum L.). J. Food Sci. Technol. 2011, 48, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Bhusari, S.; Muzaffar, K.; Kumar, P. Effect of carrier agents on physical and microstructural properties of spray dried tamarind pulp powder. Powder Technol. 2014, 266, 354–364. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov. Food Sci. Emerg. Technol. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- Tonon, R.V.; Baroni, A.F.; Brabet, C.; Gibert, O.; Pallet, D.; Hubinger, M.D. Water sorption and glass transition temperature of spray dried açai (Euterpe oleracea Mart.) juice. J. Food Eng. 2009, 94, 215–221. [Google Scholar] [CrossRef]
- Bhandari, B.; Howes, T. Implication of glass transition for the drying and stability of dried foods. J. Food Eng. 1999, 40, 71–79. [Google Scholar] [CrossRef]
- Truong, V.; Bhandari, B.R.; Howes, T. Optimization of co-current spray drying process of sugar-rich foods. Part I—Moisture and glass transition temperature profile during drying. J. Food Eng. 2005, 71, 55–65. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. Encycl. Anal. Chem. Appl. Theory Instrum. 2006. [Google Scholar] [CrossRef]
- Kačuráková, M.; Belton, P.S.; Wilson, R.H.; Hirsch, J.; Ebringerová, A. Hydration properties of xylan-type structures: An FTIR study of xylooligosaccharides. J. Sci. Food Agric. 1998, 77, 38–44. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panyoyai, N.; Bannikova, A.; Small, D.M.; Shanks, R.A.; Kasapis, S. Diffusion of nicotinic acid in spray-dried capsules of whey protein isolate. Food Hydrocoll. 2016, 52, 811–819. [Google Scholar] [CrossRef]
- Bashir, M.; Haripriya, S. Assessment of physical and structural characteristics of almond gum. Int. J. Biol. Macromol. 2016, 93, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Jaumot, J.; Gargallo, R.; de Juan, A.; Tauler, R. A graphical user-friendly interface for MCR-ALS: A new tool for multivariate curve resolution in MATLAB. Chemom. Intellig. Lab. Syst. 2005, 76, 101–110. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Aniesrani Delfiya, D.S.; Thangavel, K.; Natarajan, N.; Kasthuri, R.; Kailappan, R. Microencapsulation of Turmeric oleoresin by spray drying and in vitro release studies of microcapsules. J. Food Process Eng. 2015, 38, 37–48. [Google Scholar] [CrossRef]
- Archaina, D.; Vasile, F.; Jiménez-Guzmán, J.; Alamilla-Beltrán, L.; Schebor, C. Physical and functional properties of roselle (Hibiscus sabdariffa L.) extract spray dried with maltodextrin-gum arabic mixtures. J. Food Process. Preserv. 2019, 43, e14065. [Google Scholar] [CrossRef]
- Johnstone, C.; Hendry, C.; Farley, A.; McLafferty, E. The digestive system: Part 1. Nurs. Stand. (2014+) 2014, 28, 37. [Google Scholar] [CrossRef]
- Pedersen, A.M.; Bardow, A.; Jensen, S.B.; Nauntofte, B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. 2002, 8, 117–129. [Google Scholar] [CrossRef]
- Chmiel, T.; Saputro, I.E.; Kusznierewicz, B.; Bartoszek, A. The impact of cooking method on the phenolic composition, total antioxidant activity and starch digestibility of rice (Oryza sativa L.). J. Food Process. Preserv. 2018, 42, e13383. [Google Scholar] [CrossRef]





| Stock Concentration | SSF, pH 7 | SGF, pH 3 | SIF, pH 7 | |
|---|---|---|---|---|
| Constituent | Concentration | |||
| mol/100 mL | mmol/100 mL | mmol/100 mL | mmol/100 mL | |
| KCl | 0.05 | 1.51 | 0.69 | 0.68 |
| KH2PO4 | 0.05 | 0.37 | 0.09 | 0.08 |
| NaHCO3 | 0.1 | 1.36 | 2.5 | 8.5 |
| NaCl | 0.2 | - | 4.72 | 3.84 |
| MgCl2(H2O)6 | 0.015 | 0.015 | 0.01 | 0.033 |
| (NH4)2CO3 | 0.05 | 0.006 | 0.05 | - |
| Properties | P55 | P73 | P91 |
|---|---|---|---|
| Moisture content (%) | 5.17 ± 0.05 a | 5.25 ± 0.08 a | 5.57 ± 0.14 b |
| aw | 0.15 ± 0.01 a | 0.18 ± 0.03 ab | 0.22 ± 0.04 b |
| ρb (g/mL) | 0.507 ± 0.025 a | 0.555 ± 0.005 ab | 0.583 ± 0.005 b |
| ρt (g/mL) | 0.628 ± 0.018 a | 0.689 ± 0.014 b | 0.689 ± 0.001 b |
| Dissolution time (s) | 47.5 ± 3.5 a | 36.5 ± 2.1 b | 25.5 ± 0.7 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Khoo, S.L.A.; Swedlund, P.; Ogawa, Y.; Shan, Y.; Quek, S.Y. Fabrication of Spray-Dried Microcapsules Containing Noni Juice Using Blends of Maltodextrin and Gum Acacia: Physicochemical Properties of Powders and Bioaccessibility of Bioactives during In Vitro Digestion. Foods 2020, 9, 1316. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9091316
Zhang C, Khoo SLA, Swedlund P, Ogawa Y, Shan Y, Quek SY. Fabrication of Spray-Dried Microcapsules Containing Noni Juice Using Blends of Maltodextrin and Gum Acacia: Physicochemical Properties of Powders and Bioaccessibility of Bioactives during In Vitro Digestion. Foods. 2020; 9(9):1316. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9091316
Chicago/Turabian StyleZhang, Chuang, Siew Lin Ada Khoo, Peter Swedlund, Yukiharu Ogawa, Yang Shan, and Siew Young Quek. 2020. "Fabrication of Spray-Dried Microcapsules Containing Noni Juice Using Blends of Maltodextrin and Gum Acacia: Physicochemical Properties of Powders and Bioaccessibility of Bioactives during In Vitro Digestion" Foods 9, no. 9: 1316. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9091316