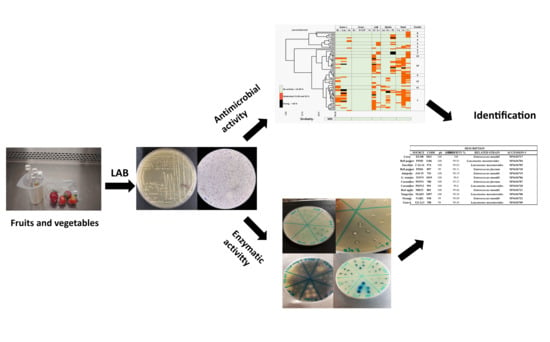
Selection of Lactic Acid Bacteria Isolated from Fresh Fruits and Vegetables Based on Their Antimicrobial and Enzymatic Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of Lactic Acid Bacteria
2.2. Antimicrobial Activity of LAB Cell-Free Supernatants
2.3. Enzymatic Capacity
2.4. Biofilm Formation
2.5. Molecular Identification of LAB
2.5.1. DNA Extraction
2.5.2. Amplification of the 16s rDNA
2.5.3. Sequencing
2.6. Statistical Analysis
3. Results
3.1. Lactic Acid Bacteria Isolation
3.2. Antimicrobial Activity of LAB Cell-Free Supernatants
3.3. Enzymatic and Adhesion Capacity
3.4. Molecular Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Borrero, J.; Kelly, E.; O’Connor, P.; Kelleher, P.; Scully, C.; Cotter, P.; Mahony, J.; Van Sinderen, D. Plantaricyclin A, a novel circular bacteriocin produced by Lactobacillus plantarum NI326: Purification, characterization, and heterologous production. Appl. Environ. Microbiol. 2018, 84, e018101-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fessard, A.; Remize, F. Genetic and technological characterization of lactic acid bacteria isolated from tropically grown fruits and vegetables. Int. J. Food Microbiol. 2019, 301, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Garzón, K.; Ortega, C.; Tenea, G.N. Characterization of bacteriocin-producing lactic acid bacteria isolated from native fruits of Ecuadorian Amazon. Pol. J. Microbiol. 2017, 66, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.; Tabanelli, G.; Mantanari, C.; Gardini, F.; Lanciotti, R. Lactic acid bacteria and natural antimicrobials to improve the safety and shelf-life of minimally processed sliced apples and lamb’s lettuce. Food Microbiol. 2015, 47, 74–84. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Kuo, C.; Chen, Y.; Ho, S.; Yanagida, F. Leucocin C-607, a Novel Bacteriocin from the Multiple-Bacteriocin-Producing Leuconostoc pseudomesenteroides 607 Isolated from Persimmon. Probiotics Antimicrob. Proteins 2018, 10, 148–156. [Google Scholar] [CrossRef]
- Liao, X.; Guo, L.; Ye, Z.; Qiu, L.; Gu, F.; Lin, J. Use of autochthonous lactic acid bacteria starters to ferment mango juice for promoting its probiotic roles. Prep. Biochem. Biotechnol. 2016, 46, 399–405. [Google Scholar] [CrossRef]
- Luz, C.; Ferrer, J.; Mañes, J.; Meca, G. Toxicity reduction of ochratoxin A by lactic acid bacteria. Food Chem. Toxicol. 2018, 112, 60–66. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Sáez, G.; Saavedra, L.; Hebert, E.; Zárate, G. Identification and biotechnological characterization of lactic acid bacteria isolated from chickpea sourdough in northwestern Argentina. LWT-Food Sci. Technol. 2018, 93, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Wang, X.; Cui, M.; Wang, Y.; Jiao, Z.; Tan, Z. Ensilage of oats and wheatgrass under natural alpine climatic conditions by indigenous lactic acid bacteria species isolated from high-cold areas. PLoS ONE 2018, 13, e0192368. [Google Scholar] [CrossRef] [Green Version]
- Carrizo, S.; Montes de Oca, C.; Hébert, M.; Saavedra, L.; Vignolo, G.; LeBlanc, J.G.; Rollán, G. Lactic acid bacteria from andean grain Amaranth: A source of vitamins and functional value enzymes. J. Mol. Microbiol. Biotechnol. 2017, 27, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Verni, M.; Wang, C.; Montemurro, M.; De Angelis, M.; Katina, K.; Rizzello, C.; Coda, R. Exploring the microbiota of faba bean: Functional characterization of lactic acid bacteria. Front. Microbiol. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sáez, G.; Hébert, E.; Saavedra, L.; Zárate, G. Molecular identification and technological characterization of lactic acid bacteria isolated from fermented kidney beans flours (Phaseolus vulgaris L. and P. coccineus) in northwestern Argentina. Food Res. Int. 2017, 102, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Domingos-Lopes, M.; Lamosa, P.; Stanton, C.; Ross, R.; Silva, C. Isolation and characterization of an exopolysaccharide-producing Leuconostoc citreum strain from artisanal cheese. Lett. Appl. Microbiol. 2018, 67, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Konkit, M.; Kim, M. Activities of amylase, proteinase, and lipase enzymes from Lactococcus chungangensis and its application in dairy products. J. Dairy Sci. 2016, 99, 4999–5007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unban, K.; Kanpiengjai, A.; Takata, G.; Uechi, K.; Lee, W.; Khanongnuch, C. Amylolytic Enzymes Acquired from L-Lactic Acid Producing Enterococcus faecium K-1 and Improvement of Direct Lactic Acid Production from Cassava Starch. Appl. Biochem. Biotechnol. 2017, 183, 155–170. [Google Scholar] [CrossRef]
- Ouattara, H.; Ouattara, H.; Droux, M.; Reverchon, S.; Nasser, W.; Niamke, S. Lactic acid bacteria involved in cocoa beans fermentation from Ivory Coast: Species diversity and citrate lyase production. Int. J. Food Microbiol. 2017, 256, 11–19. [Google Scholar] [CrossRef]
- Michalak, M.; Gustaw, K.; Waśko, A.; Polak-Berecka, M. Composition of lactic acid bacteria during spontaneous curly kale (Brassica oleracea var. sabellica) fermentation. Microbiol. Res. 2018, 206, 121–130. [Google Scholar] [CrossRef]
- Mac Faddin, J.F. Biochemical Tests for Identification of Medical Bacteria, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Inglin, R.; Stevens, M.; Meile, L.; Lacroix, C.; Meile, L. High-throughput screening assays for antibacterial and antifungal activities of Lactobacillus species. J. Microbiol. Methods 2015, 114, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.B.; Khoo, B.Y.; Sasidharan, S.; Piyawattanametha, W.; Kim, H.; Khemthongcharoen, N.; Ang, M.Y.; Chuah, L.O.; Liong, M.T. Inhibition of Staphylococcus aureus by crude and fractionated extract from lactic acid bacteria. Benef. Microbes 2015, 6, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Pailin, T.; Kang, D.; Schmidt, K.F.D. Detection of extracellular bound proteinase in EPS-producing lactic acid bacteria cultures on skim milk agar. Lett. Appl. Microbiol. 2001, 33, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Abbasiliasi, S.; Tan, J.S.; Ibrahim, T.A.; Ramanan, R.N.; Vakhshiteh, F.; Mustafa, S.; Ling, T.C.; Rahim, R.A.; Ariff, A.B. In vitro assessment of Pediococcus acidilactici Kp10 for its potential use in the food industry. BMC Microbiol. 2017, 17, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempler, G.M.; McKay, L.L. Improved medium for detection of citrate-fermenting Streptococcus lactis subsp. diacetylactis. Appl. Environ. Microbiol. 1980, 39, 926–927. [Google Scholar] [CrossRef] [Green Version]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Pospiech, A.; Neumann, B. A versatile quick-prep of genomic DNA from Gram-positive bacteria. Trends Genet. 1995, 11, 217–218. [Google Scholar] [CrossRef]
- Liu, C.; Gong, F.; Li, X.; Li, H.; Zhang, Z.; Feng, Y.; Nagano, H. Natural populations of lactic acid bacteria in douchi from Yunnan Province, China. J. Zhejiang Univ. Sci. B 2012, 13, 298–306. [Google Scholar] [CrossRef] [Green Version]
- Salvucci, E.; LeBlanc, J.G.; Pérez, G. Technological properties of Lactic acid bacteria isolated from raw cereal material. LWT-Food Sci. Technol. 2016, 70, 185–191. [Google Scholar] [CrossRef]
- Abriouel, H.; Benomar, N.; Cobo, A.; Caballero, N.; Fernández Fuentes, M.A.; Pérez-Pulido, R.; Gálvez, A. Characterization of lactic acid bacteria from naturally-fermented Manzanilla Aloreña green table olives. Food Microbiol. 2012, 32, 308–316. [Google Scholar] [CrossRef]
- Devriese, L.; Baele, M.; Butaye, P. The genus enterococcus: Taxonomy. In The Prokariotes, a Handbook on the Biology of Bacteria. Symbiotic Associations, Biotechnology, Applied Microbiology, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer Science + Business Media Inc.: New York, NY, USA, 2006; Volume 4. [Google Scholar]
- Liu, W.; Pang, H.; Zhang, H.; Cai, Y. Biodiversity of lactic acid bacteria. In Lactic Acid Bacteria: Fundamentals and Practice Biodiversity of Lactic Acid Bacteria; Zhang, H., Cai, Y., Eds.; Springer Science+business Media Dordrecht: Berlin, Germany, 2014. [Google Scholar] [CrossRef]
- Trias, R.; Bañeras, L.; Badosa, E.; Montesinos, E. Bioprotection of Golden Delicious apples and Iceberg lettuce against foodborne bacterial pathogens by lactic acid bacteria. Int. J. Food Microbiol. 2008, 123, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Monika; Savitri; Kumar, V.; Kumari, A.; Angmo, K.; Bhalla, T. Isolation and characterization of lactic acid bacteria from traditional pickles of Himachal Pradesh, India. J. Food Sci. Technol. 2017, 54, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, Y.; Lu, H.M.; Li, D.T.; Zhang, Z.L.; Tang, Z.X.; Shi, L.E. Antimicrobial activity and safety evaluation of Enterococcus faecium KQ 2.6 isolated from peacock feces. BMC Biotechnol. 2015, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venegas-Ortega, M.G.; Flores-Gallegos, A.C.; Aguilar, C.N.; Rodríguez-Herrera, R.; Martínez-Hernández, J.L.; Nevárez-Moorillón, G.V. Multi-Functional Potential of Presumptive Lactic Acid Bacteria Isolated from Chihuahua Cheese. Foods 2020, 9, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comasio, A.; Harth, H.; Weckx, S.; De Vuyst, L. The addition of citrate stimulates the production of acetoin and diacetyl by a citrate-positive Lactobacillus crustorum strain during wheat sourdough fermentation. Int. J. Food Microbiol. 2019, 289, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Sonar, N.R.; Halami, P.M. Phenotypic identification and technological attributes of native lactic acid bacteria present in fermented bamboo shoot products from North-East India. J. Food Sci. Technol. 2014, 51, 4143–4148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Żukiewicz-Sobczak, W.; Wróblewska, P.; Adamczuk, P.; Silny, W. Probiotic lactic acid bacteria and their potential in the prevention and treatment of allergic diseases. Cent. Eur. J. Immunol. 2014, 39, 104–108. [Google Scholar] [CrossRef]
- Nediani, M.; García, L.; Saavedra, L.; Martínez, S.; López Alzogaray, S.; Fadda, S. Adding Value to Goat Meat: Biochemical and Technological Characterization of Autochthonous Lactic Acid Bacteria to Achieve High-Quality Fermented Sausages. Microorganisms 2017, 5, 26. [Google Scholar] [CrossRef]
- Serio, A.; Chávez-López, C.; Paparella, A.; Suzzy, G. Evaluation of metabolic activities of enterococci isolated from Pecorino Abruzzese cheese. Int. Dairy J. 2010, 20, 459–464. [Google Scholar] [CrossRef]
- Taboada, N.; Van Nieuwenhove, C.; López Alzogray, S.; Medina, R. Influence of autochthonous cultures on fatty acid composition, esterase activity and sensory profile of Argentinean goat cheeses. J. Food Compos. Anal. 2015, 40, 86–94. [Google Scholar] [CrossRef]
- Laroute, V.; Tormo, H.; Couderc, C.; Mercier-Bonin, M.; Le Bourgeois, P.; Cocaign-Bousquet, M.; Daveran-Mingot, M. From Genome to Phenotype: An Integrative Approach to Evaluate the Biodiversity of Lactococcus lactis. Microorganisms 2017, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Martino, G.; Quintana, I.; Espariz, M.; Blancato, V.; Magni, C. Aroma compounds generation in citrate metabolism of Enterococcus faecium: Genetic characterization of type I citrate gene cluster. Int. J. Food Microbiol. 2016, 218, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.R.; Koijam, K. Exopolysaccharide production by a lactic acid bacteria, Leuconostoc lactis isolated from ethnically fermented beverage. Natl. Acad. Sci. Lett. 2014, 37, 59–64. [Google Scholar] [CrossRef]
- Kubota, H.; Senda, S.; Nomura, N.; Tokuda, H.; Uchiyama, H. Biofilm Formation by Lactic Acid Bacteria and Resistance to Environmental Stress. J. Biosci. Bioeng. 2008, 106, 381–386. [Google Scholar] [CrossRef] [PubMed]



| Indicator | ATCC | Incubation Conditions |
|---|---|---|
| Bacillus cereus | 11,778 | Aerobiosis, 37 °C, 24 h |
| Listeria monocytogenes | 19,114 | |
| Staphylococcus aureus | 25,923 | |
| Escherichia coli | 35,218 | |
| Escherichia coli O157:H7 | 43,888 | |
| Salmonella Typhimurium | 14,028 | |
| Lactococcus lactis | Commercial | Anaerobiosis, 26 °C, 24 h |
| Lacticaseibacillus casei comb.nov. | Commercial | |
| Aspergillus niger | BUAP 1 collection | Aerobiosis, 26 °C, 48 h |
| Penicillium expansum | UDLAP 2 collection | |
| Fusarium oxysporum | UDLAP 2 collection | |
| Candida albicans | 10,231 | Aerobiosis, 26 °C, 24 h |
| Saccharomyces cerevisiae | 9763 | |
| Candida tropicalis | 1369 |
| Sample | N° of Samples | N° Isolations/Shape | Sample | N° of Samples | N° Isolations/Shape |
|---|---|---|---|---|---|
| Red Apple | 15 | 4 coccoid | Zucchini | 15 | 2 rod, 2 coccoid |
| Green Apple | 15 | 6 coccoid | Green Tomato | 12 | 2 cocci, 2 coccoid |
| Pear | 6 | 3 rod, 1 coccoid | Grape | 3 | 1 rod |
| Pomegranate | 3 | 2 cocci | Orange | 6 | 1 coccus, 1 rod, 3 coccoid |
| Bell Pepper | 6 | 3 coccoid | Peach | 6 | 3 rod, 1 coccoid |
| Jalapeño Chili | 18 | 1 coccus, 5 coccoid | Guava | 15 | 2 cocci, 6 coccoid |
| Chilaca Chili | 18 | 2 cocci, 3 rod, 5 coccoid | Corn | 9 | 2 rod, 4 coccoid |
| Mandarin | 3 | coccoid | Lettuce | 6 | 2 coccoid |
| Cucumber | 12 | 2 coccoid | Nopal flower | 3 | 1 coccoid |
| Soybean | 3 | 1 coccoid |
| Enzymatic Activity | Protease | Lipase | Citrate | Amylase | Adhesion |
|---|---|---|---|---|---|
| No activity | 30 (39) | 62 (81.6) | 40 (52.6) | 71 (93.4) | 35 (46) |
| Medium | 26(34) | 11 (14.5) | 13 (17) | 3 (4) | 17 (22) |
| High | 20 (26) | 3 (4) | 23 (30) | 2 (2.6) | 24 (31.6) |
| Total positive | 46 (60.5) | 14 (18.4) | 36 (47) | 5 (6.6) | 41 (54) |
| Total sample | 76 | 76 | 76 | 76 | 76 |
| SOURCE | CODE | ADHESION |
|---|---|---|
| Corn | ELO8 | ++ |
| Bell pepper | PIM4 | ++ |
| Cucumber | PEP12 | ++ |
| Jalapeño | JAV15 | + |
| Red apple | MR15 | + |
| Tangerine | MAD3 | +++ |
| Orange | NAR1 | +++ |
| Guava | GUA13 | ++ |
| SOURCE | CODE | Selection Criteria | Pb 1 | QUERY COVER % | IDENTITY % | RELATED STRAIN | ACCESSION # |
|---|---|---|---|---|---|---|---|
| Corn | ELO8 | a, p, l, c, e | 1011 | 100 | 100 | Enterococcus mundtii | MN636717 |
| Bell pepper | PIM5 | a | 1106 | 100 | 99.91 | Leuconostoc mesenteroides | MN636704 |
| Zucchini | CAL14 | a | 574 | 100 | 99.83 | Leuconostoc mesenteroides | MN636705 |
| Bell pepper | PIM4 | a, p, l, c, e | 897 | 99 | 99.11 | Enterococcus faecium | MN636718 |
| Jalapeño | JAV15 | a, p, l, c | 743 | 100 | 99.19 | Enterococcus mundtii | MN636719 |
| G. tomato | TOV9 | a, l, c, e | 1019 | 100 | 99.8 | Enterococcus mundtii | MN636706 |
| Cucumber | PEP11 | a | 780 | 100 | 95.27 | Enterococcus faecium | MN636707 |
| Cucumber | PEP12 | a, p, l, c, e | 991 | 100 | 99.6 | Leuconostoc mesenteroides | MN636720 |
| Red apple | MR15 | p, l, m, c e | 881 | 100 | 99.66 | Enterococcus mundtii | MN636721 |
| Tangerine | MAD3 | p, l, m, c, e | 1097 | 100 | 99.54 | Leuconostoc mesenteroides | MN636708 |
| Orange | NAR1 | p, m, c, e | 938 | 99 | 99.89 | Enterococcus mundtii | MN636722 |
| Guava | GUA13 | p, l, c, e | 708 | 99 | 99.43 | Leuconostoc mesenteroides | MN636709 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linares-Morales, J.R.; Cuellar-Nevárez, G.E.; Rivera-Chavira, B.E.; Gutiérrez-Méndez, N.; Pérez-Vega, S.B.; Nevárez-Moorillón, G.V. Selection of Lactic Acid Bacteria Isolated from Fresh Fruits and Vegetables Based on Their Antimicrobial and Enzymatic Activities. Foods 2020, 9, 1399. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9101399
Linares-Morales JR, Cuellar-Nevárez GE, Rivera-Chavira BE, Gutiérrez-Méndez N, Pérez-Vega SB, Nevárez-Moorillón GV. Selection of Lactic Acid Bacteria Isolated from Fresh Fruits and Vegetables Based on Their Antimicrobial and Enzymatic Activities. Foods. 2020; 9(10):1399. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9101399
Chicago/Turabian StyleLinares-Morales, José Rafael, Guillermo Eduardo Cuellar-Nevárez, Blanca Estela Rivera-Chavira, Néstor Gutiérrez-Méndez, Samuel Bernardo Pérez-Vega, and Guadalupe Virginia Nevárez-Moorillón. 2020. "Selection of Lactic Acid Bacteria Isolated from Fresh Fruits and Vegetables Based on Their Antimicrobial and Enzymatic Activities" Foods 9, no. 10: 1399. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9101399